High Purity Excipients: A Simple Solution to a Complex Problem
Introduction
It wouldn’t come as a surprise to anyone that active pharmaceutical ingredients (API) come in a wide variety.
Small molecule, large molecule, peptide, monoclonal antibody; the list goes on. These molecules have the ability to cure or mitigate debilitating conditions which can change a person’s life forever.
It makes sense, then, that these ingredients are of primary importance in a formulation, and appropriate measures should be taken to maintain their stability and efficacy.
As these APIs become more complex, they also become increasingly vulnerable to a series
of different degradation pathways. Changes in pH environments can cause acidification and lead to breakdown.
Exposure to moisture can initiate hydrolysis and subsequently lead to the formation of
secondary byproducts. Residual catalyst that isn’t removed from an excipient can trigger side reactions and perpetuate degradation of not just the API, but everything else in the formulation.
The practical solution is to ensure that the used ingredients in the formulation are of the highest quality and purity. This certifies that the drug will not degrade, and that efficacy and longevity are maintained.
Polysorbates for Biopharmaceuticals
It is well documented that biopharmaceutical actives, such as proteins and nucleic acids, readily undergo breakdown when exposed to various external stresses, including, heat, pressure, purification processes, mixing.
This causes unfavorable interactions either within the protein structure or interactions
with an external surface, ultimately leading to a decreased biologic efficacy.
Additionally, these unfavorable interactions and product breakdown can be initiated through exposure to degradants found within the formulation. In this scenario, the culprit is the excipient that the therapeutic agent is formulated with.
While in minute quantities, one of the key ingredients that these agents are formulated
with are polysorbates, specifically Polysorbate 20 and Polysorbate 80.
Polysorbates are used in a wide variety of applications as a stabilization and surface adsorption prevention agent for proteins.
Its inherent biocompatibility and strong ability to maintain internal protein structure also makes it a favorable choice over other stabilizers, like human serum albumin (HSA) and disaccharides1.
However, polysorbates are also notorious for undergoing autooxidation. While the exact breakdown mechanism of these components is still unclear, it is theorized that the primary means are via acid or base-catalyzed hydrolysis or stress-initiated breakdown into aldehyde
and acid subunits2.
These subunits not only further propagate the breakdown of the polysorbate, but also
interfere with stability of the active ingredient.
For this reason, it is imperative that the highest purity ingredients are used in these kinds of sensitive applications. Croda’s Super Refined™ Polysorbate 20 and Super Refined™ Polysorbate 80 has the low impurity and high stability profile that’s required of these applications.
Super Refined Polysorbates solubilise and stabilise the most sensitive APIs (active pharmaceutical ingredients) across dosage forms including injectable and oral.
Super Refining removes polar impurities (including primary and secondary oxidation products) from an excipient without altering its chemical composition, helping to reduce negative API interaction and degradation.
The Real Benefits of Purity
The main concern with oxidative impurities in excipients doesn’t just pertain to active stability. Instability of the ingredient correlates to a number of concerns related to formulation and drug delivery.
Select impurities are known to be cellular irritants, inhibiting sufficient drug delivery and even inducing pain at the site of application.
Small molecular weight impurities like formaldehyde can interfere with supplemental delivery vessels like gelatin capsules, altering or even preventing formulation release.
As can be seen below Super Refined Polysorbate 20 contained approximately 46% less formaldehyde as compared to standard compendial grade polysorbate 20 (Figure 1).
Likewise, when comparing Super Refined Polysorbate 80, it was observed that Super
Refined Polysorbate 80 contains approximately 81% less formaldehyde than standard grade Polysorbate 80. (Figure 2)
[caption id="attachment_153226" align="aligncenter" width="471"]
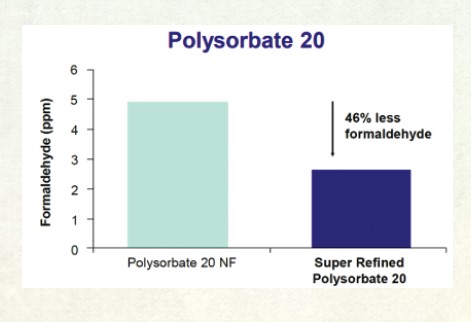
Figure 1: A graph showing the difference in formaldehyde content between Super Refined Polysorbate 20 and standard pharmaceutical grade polysorbate 20.[/caption]
[caption id="attachment_153227" align="aligncenter" width="452"]

Figure 2: A graph showing the difference in formaldehyde content between Super Refined Polysorbate 80 and standard pharmaceutical grade polysorbate 80.[/caption]
It is not just that a product should be synthesized with purity in mind, but also that the product should be handled with purity in mind.
This isn’t just the case with polysorbates, but with all ingredients used in the manufacturing of pharmaceuticals. Proper precautions, such as critically controlling the cleaning protocols of vessels and inerting packaging and filling environments are crucial to excipient stability and, ultimately, product performance.
Conclusion
As conditions evolve, so do active pharmaceutical ingredients. Complexity in design and structure lead to tailored and efficacious delivery for those who need it most.
Drug degradation can have toxicological effects in many instances, and it is imperative that this mechanism be minimized as much as possible.
The best solution to this is to ensure that appropriate ingredients, both high in quality and purity, are chosen and used throughout the entire drug product lifecycle.
Croda’s Super Refined™ range of excipients are a testament to the term purity. With a wide range of ingredients available across the globe, coupled with expertise in numerous formulation and drug delivery areas, Croda offers a complete line of solutions which helps bring innovative breakthrough therapies to market.
Super Refined™ excipients are extensively purified to remove primary and secondary oxidation products, including aldehydes, hydroperoxides, and ketones, as well as residual catalyst from the synthetic process.
This allows for a cleaner and clearer product that has a prolonged stability and shelf life,
as well as allows for better stability of any active ingredient that is solubilized in it.
Gizem Uzun
Sales Representative - Pharma
Croda Türkiye
References
1. Arsiccio, Andrea, and Roberto Pisano. “Surfactants as Stabilizers for Biopharmaceuticals: An Insight into the Molecular Mechanisms for Inhibition of Protein Aggregation.” European Journal of Pharmaceutics and Biopharmaceutics, vol. 128, 9 Apr. 2018, pp. 98–106., doi:10.1016/j.ejpb.2018.04.005.
2. Kerwin, Bruce A. “Polysorbates 20 and 80 Used in the Formulation of Protein Biotherapeutics: Structure and Degradation Pathways.” Journal of Pharmaceutical Sciences, vol. 97, no. 8, 29 Jan. 2016, pp. 2924–2935., doi:10.1002/jps.21190.
Non-warranty CPAR001v1 EN
The information in this publication is believed to be accurate and is given in good faith, but no representation or warranty as to its completeness or accuracy is made. Suggestions for uses or applications are only opinions. Users are responsible for determining the suitability of these products for their own particular purpose.
No representation or warranty, expressed or implied, is made with respect to information or products including, without limitation, warranties of merchantability, fitness for a particular purpose, non-infringement of any third party patent or other intellectual property rights including, without limit, copyright, trademark and designs. Any trademarks identified herein are trademarks of the Croda group of companies. ©2020 Croda Inc.

 Figure 1: A graph showing the difference in formaldehyde content between Super Refined Polysorbate 20 and standard pharmaceutical grade polysorbate 20.[/caption]
[caption id="attachment_153227" align="aligncenter" width="452"]
Figure 1: A graph showing the difference in formaldehyde content between Super Refined Polysorbate 20 and standard pharmaceutical grade polysorbate 20.[/caption]
[caption id="attachment_153227" align="aligncenter" width="452"] Figure 2: A graph showing the difference in formaldehyde content between Super Refined Polysorbate 80 and standard pharmaceutical grade polysorbate 80.[/caption]
It is not just that a product should be synthesized with purity in mind, but also that the product should be handled with purity in mind.
This isn’t just the case with polysorbates, but with all ingredients used in the manufacturing of pharmaceuticals. Proper precautions, such as critically controlling the cleaning protocols of vessels and inerting packaging and filling environments are crucial to excipient stability and, ultimately, product performance.
Figure 2: A graph showing the difference in formaldehyde content between Super Refined Polysorbate 80 and standard pharmaceutical grade polysorbate 80.[/caption]
It is not just that a product should be synthesized with purity in mind, but also that the product should be handled with purity in mind.
This isn’t just the case with polysorbates, but with all ingredients used in the manufacturing of pharmaceuticals. Proper precautions, such as critically controlling the cleaning protocols of vessels and inerting packaging and filling environments are crucial to excipient stability and, ultimately, product performance.