Clever Combinations Protecting pH-neutral Formulations the Natural Way
Bio-based 1,2-alkanediols are ideal ingredients for modern natural cosmetics: they are multifunctional, gentle to the skin and environmentally friendly. Moreover, formulations containing 1,2-alkanediols are typically self-preserving, even at neutral pH. Clever combinations of 1,2-alkanediols with other bio-based additives further increase the mildness of formulations and reduces their cost.
In addition, liquid premixes of 1,2 alkanediols and suitable additives also simplify the application and increase productivity. However, to achieve a successful outcome requires making the right choice between different 1,2-alkanediols and different additives. This article shares guidance on the choices available to protect natural cosmetics safely and cost-effectively and includes supportive evidence.
Modern Cosmetics
With today’s focus on well-being, consumers are increasingly demanding natural and responsibly-made cosmetics.
Natural beauty products, and those with ‘clean’ label certifications like ‘vegan’ or ‘non-GMO’, are very much in vogue with ‘Back to basics’ as the new ideal. As a result, cosmetics launched with “natural” and “sustainable” claims have seen an average annual increase of 35% over the past 10 years.[1] And of course, modern cosmetics of today should
be mild to the skin and contain only undisputed ingredients that are necessary for effectiveness. In this context, public scrutiny of preservatives has grown.
Most conventional preservatives are made from fossil carbon, and some of which are harmful to the environment. Several ingredients, such as formaldehyde donors, methylisothiazolinones or parabens, have come under media fire for their controversial side effects. Safe and effective natural alternatives are thus in high demand. Currently, many natural cosmetics are preserved using carboxylic acids.
But these are only effective in acidic environments, which may irritate the skin. Another common natural preservative is ethanol, known to be volatile and flammable and not accepted in all regions of the world.
Consequently, there are only a few solutions for protecting natural cosmetics with a neutral pH-value between 5.5 and 7. Yet, it is this pH range that is especially mild and skin-friendly.
Bio-based and Sustainable
Choosing to use bio-based 1,2-alkanediols to protect natural cosmetics is a relatively novel option. Two of these products have recently become available offering significant advantages over ethanol: bio-based Pentylene Glycol and bio-based Caprylyl Glycol are skin-moisturizing, odourless, non-volatile and non-flammable.
lso, compared to carboxylic acids, these 1,2-alkanediols are milder and protect formulations even in the most skin-friendly neutral pH-range. While 1,2-alkanediols occur in nature, they are only found as metabolites of certain microorganisms. Therefore, commercially available products are man-made, either from fossil carbon or from vegetable feedstock. The bio-based versions are produced in accordance with the principles of green chemistry.[2] Bio-based Pentylene Glycol is derived from hemicellulose, while bio-based Caprylyl Glycol is obtained from natural oils (Figure 1).
Both ingredients meet COSMOS and NATRUE eco-certification standards, and both have a natural origin index of 1 according to ISO 16128. Their multifunctionality as skin humectants, conditioners and emollients fits well with current clean beauty concepts and can replace at once a number of conventional ingredients. This shortens the number of ingredients to be procured.
[caption id="attachment_134341" align="aligncenter" width="351"]

Figure 1. Routes to obtain sustainable multifunctional ingredients via Green Chemistry. [2][/caption]
1,2-Alkanediols Need Help
It is possible to protect cosmetic formulations using only 1,2-alkanediols, such as bio-based Pentylene Glycol. However, it requires concentrations of about 4-5% Pentylene Glycol to pass a standard challenge test according to ISO 11930 or the European Pharmacopoeia norm. As this can be costly, antimicrobial efficacy can be significantly offset by combining Pentylene Glycol with small amounts of other multifunctional additives. Such combinations
typically reduce the required amount of Pentylene Glycol to about 1.5- 2.5%.
The underlying effect is called “boosting,” which refers to the ability of Pentylene Glycol to disrupt microbial cell membranes: Different antimicrobial agents assist each other in penetrating the microbes, so that lower than usual concentrations are sufficient for
complete preservation. Being miscible with water, Pentylene Glycol also stabilizes other antimicrobial ingredients in the aqueous phase, where most microorganisms reside.
Finally, being truly multifunctional, Pentylene Glycol moisturizes the skin, helps to disperse pigments, assists in the hydration of gellants and creates a smooth and non-greasy skin feel.[3]
Compared to the water-soluble Pentylene Glycol, its longer-chain relative Caprylyl Glycol is more lipophilic. Its solubility in water is limited to 0.75%, but use levels of only about 0.5% Caprylyl Glycol are typically sufficient to completely protect a cosmetic product from microbial infestation. Its remarkable antimicrobial efficacy makes biobased Caprylyl Glycol one of the safest and most cost-effective solutions for creating self-preserving natural formulations.[4] However, Caprylyl Glycol is a waxy solid that melts at 30- 35°C. The process of melting can take a long time, and the molten product has a high tendency
to form supercooled melts, making the freezing point unpredictable.
Finally, the material increases in volume as it freezes, which can cause drums and pipes to burst. All this makes Caprylyl Glycol a capricious ingredient that is quite inconvenient to handle. Combining biobased Caprylyl Glycol with other components in a stable liquid blend may help to improve its handling while retaining its benefits. Thanks to some boosting effects, the concentration of the 1,2-alkanediol can be reduced to about 0.2-0.4%, which can also help to reduce the cost and increase the mildness of the final formulation.
The most straightforward approach to stabilizing bio-based Caprylyl Glycol in a liquid form is to combine it with inert additives such as water or glycerol. Interestingly, the addition of water to a mixture of Caprylyl Glycol and glycerol does not lead to phase separation. Instead, stable liquids with melting points below 20°C are formed, consisting of up to 75-80% Caprylyl Glycol. In this form, Caprylyl Glycol is easy to handle and suitable for cold processing.
Simple storage at room temperature is sufficient to keep it liquid. This saves time and energy and allows safe handling without heating or local air extraction. All the positive aspects of bio-based Caprylyl Glycol are maintained, such as low odour, low cost in use and essentially no pH restriction.
Performance Under Realistic Conditions
For the efficacy screening, each of the two bio-based 1,2-alkanediols Pentylene Glycol and Caprylyl Glycol was combined separately with each of the two biobased additives Phenylpropanol and Glyceryl Caprylate/Caprate. The mixtures were formulated into storage stable liquid blends (Table 1).
For comparison, the efficacy of the two bio-based 1,2-alkanediols were also tested separately without additives. The biobased Caprylyl Glycol was finally also applied in
its physically stabilized liquid form (“E-Leen 8”). Table 1 summarizes the different combinations tested. The ingredients listed in Table 1 were evaluated for their antimicrobial performance in three different personal care products, thus covering common mass-market applications:
• Natural O/W Emulsion (Table 2 A),
• Sulphate-free Shampoo (Table 2 B),
• Eco-friendly, Cellulose-based Wet-Wipes (Figure 3).
The blends and single 1,2-alkanediols were tested at different use-levels and pH-values in microbial challenge tests according to the norm ISO 11930. Each product was separately infected with five different microbes (one yeast, one mould, three different bacteria). The germ counts were determined after 7, 14 and 28 days. Depending on the reduction of the germ counts, the formulations were found to either fulfil criteria A (fully protected), criteria B (partly protected) or none of the criteria (failed, not protected). Table 3 shows a summary of the results obtained for the O/W emulsion and the sulphate-free shampoo.
[caption id="attachment_134342" align="aligncenter" width="731"]

Table 1. Combinations with bio-based additives and bio-based 1,2-alkanediols.[/caption]
Results and Discussion
The test results also show that Pentylene Glycol and Caprylyl Glycol can be considered as complementary ingredients. Each of the two ingredients is particularly suitable for certain applications. The use-levels of 2-3% for the Pentylene Glycol-based blends E-Leen Green A and E-Leen Green C (Table 3, entries 2 and 3) are comparatively high. These blends are therefore particularly suitable for formulations that benefit from the multifunctionality of the Pentylene Glycol, such as its skin-humectant, dispersing, wetting, emollient or solubilising properties. Since Pentylene Glycol is a water soluble 1,2-alkanediol, it has a low tendency to migrate into oil-phases or to get captured inside micelles.
Indeed, it can even stabilize lipophilic ingredients inside water phases. All this makes blends based on Pentylene Glycol particularly suitable for formulations that are rich
in oils or contain high concentrations of surfactants.
[caption id="attachment_134343" align="aligncenter" width="746"]
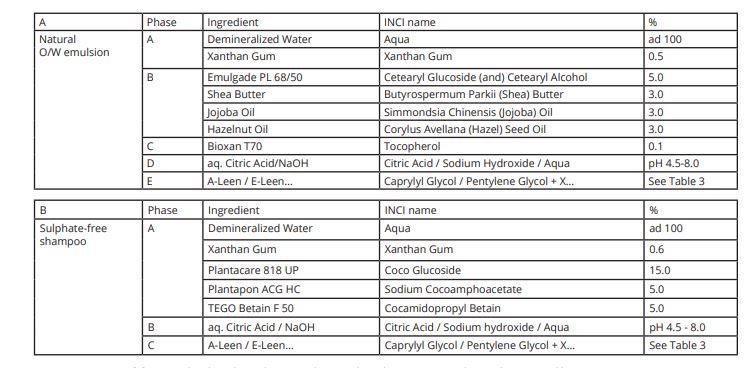
Table 2. Natural O/W emulsion and sulphate-free shampoo, used for microbial challenge tests.[/caption]
[caption id="attachment_134344" align="aligncenter" width="607"]

Table 3. Results of microbial challenge tests according to ISO 11930[/caption]
Consequently, the two tested blends containing Pentylene Glycol proved to be the most versatile during the challenge tests. They were able to protect the O/W emulsion as well
as the sulphate-free shampoo at acceptable use-levels (Table 3, entries 2 and 3). The tested sulphate-free shampoo is solely based on mild surfactants. Such kind of formulations can be very challenging to preserve. In contrast to this, many common cleaning products are based on sulphate or sulfonate surfactants.
These already provide an inherent antimicrobial effect. Consequently, products containing sulphates or sulfonates are often partly self-preserving. They can therefore be protected with comparably smaller amounts of antimicrobial additives. Caprylyl Glycol proved to be a very economical protection agent during the tests.
Caprylyl Glycol offers fewer multifunctional advantages than Pentylene Glycol, mainly due to its low use-level. The O/W emulsion tested was already protected with a small amount of Caprylyl Glycol. The physically stabilized liquid form “E Leen 8” (Table 3, entry 5) was comparably effective as the pure form “A-Leen 8” (Table 3, entry 4). In addition, the liquid form was significantly easier to apply.
The “boosted” mixture E-Leen P8, additionally containing Phenylpropanol, proved to be even more effective than the pure 1,2-alkanediol. It consequently offers an increased
safety margin for demanding formulations (Table 3, entry 6). On the other hand, the skin-friendly blend E-Leen GC 8 (Table 3, entry 7) enabled the full protection of the O/W emulsion to be achieved with lower amounts of Caprylyl Glycol.
Interestingly, despite its tremendous antimicrobial activity, relatively high concentrations of Caprylyl Glycol and corresponding blends were required to protect the sulphate-free shampoo (Table 3, entries 4-7). The reason lies in the lipophilicity of Caprylyl Glycol. It is thus more easily entrapped inside surfactant micelles than the hydrophilic Pentylene Glycol. The addition of Phenylpropanol provided only a limited enhancement effect, also because aromatic alcohols tend to be partially inactivated by non-ionic surfactants. Nevertheless, the Caprylyl Glycol-based solutions can be a performant and economic choice for cleaning products based on sulphate or sulfonate surfactants.
The Challenges in Protecting Wet Wipes
The final challenge for a “green antimicrobial” is probably the protection of wet wipes. Their solid support provides a large surface area, while the juice of the wipes provides water and nutrients. All these factors strongly favour microbial growth. The more “natural” a tissue is, the more likely it can serve as food or support for certain microbes. To increase the rigor of the tests, an environmentally friendly cellulose-based airlaid tissue was used, which was kindly provided by Ascutec.[5] The applied wetwipes juice consisted of only water, a biobased detergent (NatragemTM S140 NP, Croda) and a citrate buffer (Figure 2).
This aqueous solution was protected with E-Leen 8 (1%), E-Leen P8 (1%) or E-Leen GC 8 (1%), respectively. Two weight equivalents of the wet wipe juice were applied to one weight equivalent of a dry wipe. The wipes were infected with germs and incubated in sealed plastic bags. The number of germs was counted after 7, 14, and 28 days of incubation in accordance with the ISO 11930 standard. The results of the challenge tests are summarized in Figure 2. The unpreserved wipes exhibited very high growth for
all types of microbes tested (results not shown). The wet wipes protected with E-Leen 8 and E-Leen P8 passed the challenge tests and achieved criteria A. The blend E-Leen
GC 8 also showed broad-spectrum antimicrobial activity, but requires a use-level higher than 1% to meet criteria A.
These results confirm that solutions containing biobased Caprylyl Glycol are a convenient option for producing skin-friendly wet wipes. The solutions provide skin-caring effects while creating self-preserving wipes. The use of irritating or persistent additives can thus be avoided. At the same time, these solutions are 100% bio-based, COSMOS and NATRUE compliant and readily biodegradable. Their low use levels also make them economically interesting, and their liquid form enables high-throughput production.
[caption id="attachment_134345" align="aligncenter" width="370"]

Figure 2. Formulation of wet wipe juice, and results of microbial challenge tests pf the impregnated wipes. The red bars indicate the target values for germ-count reductions after 28 days of incubation.[/caption]
[caption id="attachment_134346" align="aligncenter" width="530"]
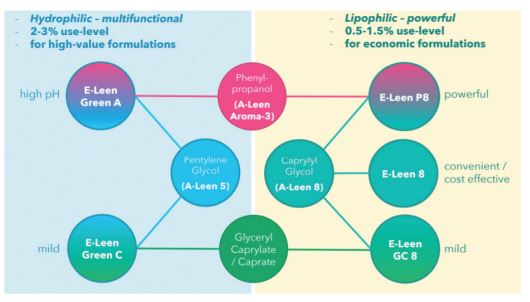
Figure 3. Bio-based blends with Pentylene Glycol and Caprylyl Glycol offer complementary
functionalities.[/caption]
Summary
The bio-based versions of the two 1,2-alkanediols “Pentylene Glycol” and “Caprylyl Glycol” prove to be versatile ingredients. In combination with other biobased additives, they are an economical, time- and cost-saving option for the production of self-preserved natural cosmetics (Figure 3). Bio-based Pentylene Glycol is generally suitable for products that benefit from its multifunctionality. Due to its high solubility in water, it is particularly useful as an additive to oil-based emulsions, giving them a lighter skin feel with faster absorption. Another positive aspect is its low tendency to get trapped in micelles, a very useful property for antimicrobial additives.
Bio-based Caprylyl Glycol is a high performance alternative suitable for the most common types of O/W-emulsions and surfactant products. Its low use-level makes it an unbeatably affordable option. Not least because of this, it is a real alternative for eco-certified wet wipes. The aromatic alcohol is resistant to high and low pH values. It gives an extra boost to antimicrobial efficacy and provides an increased margin of safety for demanding applications. Finally, it is also a safe and pleasant perfuming component that can be dissolved in water at appropriate concentrations. Its complementary counterpart, Glyceryl Caprylate/Caprate, is a very gentle ingredient that offers the ability to protect products for sensitive skin safely and cost-effectively.
Its lipophilic structure and low solubility in water suggest O/W-emulsions as the main area of application. Thanks to this novel toolbox, cosmetics manufacturers and consumers can benefit from milder products with fewer ingredients. The sustainable origin is a welcome advantage that meets today’s consumer expectations and contributes to the global challenge of creating a circular economy based on renewable materials.
References
[1] MINTEL GNPD: number of cosmetics launched worldwide in 2011 – 2020 with claim “sustainable”.
[2] P.T. Anastas, J. C. Warner, “Green chemistry: theory and practice”, Oxford University Press, 1998.
[3] N. Konaté, M. Nahrwold, ”Sustainably Sourced Pentylene Glycol – a Green Allrounder”, SOFW Journal (English Edition) 2016, 142, 44-48.
[4] J. Toliver, S. Narasimhan, “Caprylyl Glycol: A Versatile Material to Boost Preservatives”, Cosmetics & Toiletries, July/August 2018, vol. 133, N° 7, 18-23.
[5] http://www.ascutec.de/flushable-news-english/
Author
Markus Nahrwold
Technical Director
Minasolve
Translator
Gülce Çay
Sales Executive
Arerko

 Figure 1. Routes to obtain sustainable multifunctional ingredients via Green Chemistry. [2][/caption]
Figure 1. Routes to obtain sustainable multifunctional ingredients via Green Chemistry. [2][/caption]
 Table 1. Combinations with bio-based additives and bio-based 1,2-alkanediols.[/caption]
Table 1. Combinations with bio-based additives and bio-based 1,2-alkanediols.[/caption]
 Table 2. Natural O/W emulsion and sulphate-free shampoo, used for microbial challenge tests.[/caption]
[caption id="attachment_134344" align="aligncenter" width="607"]
Table 2. Natural O/W emulsion and sulphate-free shampoo, used for microbial challenge tests.[/caption]
[caption id="attachment_134344" align="aligncenter" width="607"] Table 3. Results of microbial challenge tests according to ISO 11930[/caption]
Consequently, the two tested blends containing Pentylene Glycol proved to be the most versatile during the challenge tests. They were able to protect the O/W emulsion as well
as the sulphate-free shampoo at acceptable use-levels (Table 3, entries 2 and 3). The tested sulphate-free shampoo is solely based on mild surfactants. Such kind of formulations can be very challenging to preserve. In contrast to this, many common cleaning products are based on sulphate or sulfonate surfactants.
These already provide an inherent antimicrobial effect. Consequently, products containing sulphates or sulfonates are often partly self-preserving. They can therefore be protected with comparably smaller amounts of antimicrobial additives. Caprylyl Glycol proved to be a very economical protection agent during the tests.
Caprylyl Glycol offers fewer multifunctional advantages than Pentylene Glycol, mainly due to its low use-level. The O/W emulsion tested was already protected with a small amount of Caprylyl Glycol. The physically stabilized liquid form “E Leen 8” (Table 3, entry 5) was comparably effective as the pure form “A-Leen 8” (Table 3, entry 4). In addition, the liquid form was significantly easier to apply.
The “boosted” mixture E-Leen P8, additionally containing Phenylpropanol, proved to be even more effective than the pure 1,2-alkanediol. It consequently offers an increased
safety margin for demanding formulations (Table 3, entry 6). On the other hand, the skin-friendly blend E-Leen GC 8 (Table 3, entry 7) enabled the full protection of the O/W emulsion to be achieved with lower amounts of Caprylyl Glycol.
Interestingly, despite its tremendous antimicrobial activity, relatively high concentrations of Caprylyl Glycol and corresponding blends were required to protect the sulphate-free shampoo (Table 3, entries 4-7). The reason lies in the lipophilicity of Caprylyl Glycol. It is thus more easily entrapped inside surfactant micelles than the hydrophilic Pentylene Glycol. The addition of Phenylpropanol provided only a limited enhancement effect, also because aromatic alcohols tend to be partially inactivated by non-ionic surfactants. Nevertheless, the Caprylyl Glycol-based solutions can be a performant and economic choice for cleaning products based on sulphate or sulfonate surfactants.
Table 3. Results of microbial challenge tests according to ISO 11930[/caption]
Consequently, the two tested blends containing Pentylene Glycol proved to be the most versatile during the challenge tests. They were able to protect the O/W emulsion as well
as the sulphate-free shampoo at acceptable use-levels (Table 3, entries 2 and 3). The tested sulphate-free shampoo is solely based on mild surfactants. Such kind of formulations can be very challenging to preserve. In contrast to this, many common cleaning products are based on sulphate or sulfonate surfactants.
These already provide an inherent antimicrobial effect. Consequently, products containing sulphates or sulfonates are often partly self-preserving. They can therefore be protected with comparably smaller amounts of antimicrobial additives. Caprylyl Glycol proved to be a very economical protection agent during the tests.
Caprylyl Glycol offers fewer multifunctional advantages than Pentylene Glycol, mainly due to its low use-level. The O/W emulsion tested was already protected with a small amount of Caprylyl Glycol. The physically stabilized liquid form “E Leen 8” (Table 3, entry 5) was comparably effective as the pure form “A-Leen 8” (Table 3, entry 4). In addition, the liquid form was significantly easier to apply.
The “boosted” mixture E-Leen P8, additionally containing Phenylpropanol, proved to be even more effective than the pure 1,2-alkanediol. It consequently offers an increased
safety margin for demanding formulations (Table 3, entry 6). On the other hand, the skin-friendly blend E-Leen GC 8 (Table 3, entry 7) enabled the full protection of the O/W emulsion to be achieved with lower amounts of Caprylyl Glycol.
Interestingly, despite its tremendous antimicrobial activity, relatively high concentrations of Caprylyl Glycol and corresponding blends were required to protect the sulphate-free shampoo (Table 3, entries 4-7). The reason lies in the lipophilicity of Caprylyl Glycol. It is thus more easily entrapped inside surfactant micelles than the hydrophilic Pentylene Glycol. The addition of Phenylpropanol provided only a limited enhancement effect, also because aromatic alcohols tend to be partially inactivated by non-ionic surfactants. Nevertheless, the Caprylyl Glycol-based solutions can be a performant and economic choice for cleaning products based on sulphate or sulfonate surfactants.
 Figure 2. Formulation of wet wipe juice, and results of microbial challenge tests pf the impregnated wipes. The red bars indicate the target values for germ-count reductions after 28 days of incubation.[/caption]
[caption id="attachment_134346" align="aligncenter" width="530"]
Figure 2. Formulation of wet wipe juice, and results of microbial challenge tests pf the impregnated wipes. The red bars indicate the target values for germ-count reductions after 28 days of incubation.[/caption]
[caption id="attachment_134346" align="aligncenter" width="530"] Figure 3. Bio-based blends with Pentylene Glycol and Caprylyl Glycol offer complementary
Figure 3. Bio-based blends with Pentylene Glycol and Caprylyl Glycol offer complementary