
Introduction
The combination of two powerful and sensitive analytical techniques, which are Ion Chromatography (IC) and Mass Spectrometry (MS), creates a more powerful hybrid technique, IC-MS. This multi-parameter method, which has superior properties than other analytical techniques, can detect various organic acids and inorganic anions in a single run.

Typical IC-MS System
With the high sensitivity and selectivity of MS combined with the excellent separation capacity of IC, analytes can be resolved and detected at low concentration levels, even in complex matrices and/or in the presence of interfering ions. This indicates that IC-MS is a superior combination to UV/VIS or conductivity detection.
Ion chromatography can separate different ions, or even different ionic forms of the same element (speciation) and reduce interferences from the sample matrix. Mass selective detection complemented by classical conductivity detection, guarantees to determine the identity of the analyte, and achieve lower detection limits. In the IC-MS hybrid combination where components are measured with specific m/z signals, accurate and sensitive analysis is possible even at lower chromatographic resolution.
Proper sample preparation removes interfering substances prior to IC injection and improves analyte detection limits. With full automation of this important step, manual workload is reduced, ensuring repeatable and reliable results. Such advantages are provided by Metrohm Inline Sample Preparation (MISP) techniques (e.g. Inline Ultrafiltration or Inline Dialysis). MISP enables the accurate and simple analysis of chemicals and organic solvents from the semiconductor industries, as well as water samples, liquid products from the food and beverage industry, or clinical samples such as urine.
Native IC drivers enable users to control and operate IC systems (including autosamplers and Dosino devices) within the same MS software. Metrohm offers separate drivers for Waters™ Empower™ and Agilent OpenLab CDS (Metrohm IC Driver for Empower™, Metrohm IC Driver for OpenLab CDS respectively).
In this review article, we focus on IC-MS hybrid techniques and their applications for the direct identification and quantification of organic acids and inorganic anions in different sample matrices. We will be covering the following topics:
A. Impurities in High-quality Chemicals
· Weak organic acids in NMP, methanol, ethanol, 2-propanol, and hydrogen peroxide
· Organic acids and inorganic anions in organic matrices
B. Quality Control of Beverages
· Organic acids in soft drinks
· Oxyhalide impurities in bottled water
C. Medical Applications: Organic Acids in Urine
A. IMPURITIES IN HIGH-QUALITY CHEMICALS
In manufacturing processes such as semiconductor production, solutions and equipment must be free of all kinds of contaminants to achieve the best results. For all chemicals involved, process monitoring, and strict quality control are mandatory. In addition to other parameters, the concentration of inorganic anions must remain below certain limits.
Trace levels of organic acids are considered impurities and can interfere with various industrial processes such as microchip manufacturing. The ultrapure water used for these processes must meet the highest purity standards. Additionally, organic solvents and other chemicals should contain no or very small amounts of organic acids for optimal production efficiency.
IC-MS is an appropriate analytical technique for checking high-quality chemicals for trace levels of organic acids and inorganic anions during influent inspection and process control. Owing to the automatic Metrohm Inline Matrix Elimination, ions in many different matrices can be measured with the same analysis method.
Weak Organic Acids in NMP, Methanol, Ethanol, 2-Propanol, and Hydrogen Peroxide
Trace levels of anions and weak organic acids are determined by IC-MS in 1-methyl-2-pyrolidinone (NMP), methanol, ethanol, 2-propanol, and hydrogen peroxide (H2O2). Automatic pre-concentration and matrix elimination (MISP) of samples allow robust and reproducible measurement of all components within the sample without any matrix interference. In the analyses, peak separation was performed using a Metrosep A Supp 7-250/4.0 column. Standard anions and five organic acids were separated within 30 min by a dual high-pressure gradient. To obtain more information from the samples, an MS with electrospray ionization (ESI) was coupled as a second independent detector (Figure 2) behind conductivity detection with sequential suppressor (Figure 1). The conductivity signal is advantageous for monitoring major ionic compounds. MS signal (single ion recording) is suitable for reliable identification of analytes and quantification in the µg/L range.
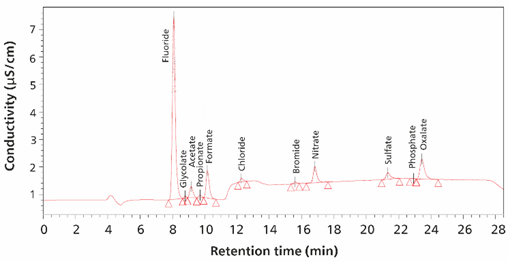
Figure 1. The conductivity signal of fluoride (400 µg/L), glycolate (40 µg/L), acetate (160 µg/L), propionate (80 µg/L), formate (160 µg/L), chloride (10 µg/L), bromide (20 µg/L), nitrate (10 µg/L), sulfate (40 µg/L), phosphate (400 µg/L) and oxalate (160 µg/L) ions are shown in a 250 µL ultrapure water sample. Separation was performed with a Metrosep ASupp 7 - 250/4.0 column (eluent-gradient: sodium carbonate/acetonitrile, flow rate 0.8 mL/minute, column temperature 45°C). The software used was Waters EmpowerTM CDS.
An example of ethanol analysis is shown in Figure 2. Acetate, propionate, oxalate, sulfate, nitrate, and chloride components were detected at concentrations ranging from 1-50 µg/L. Fluoride, glycolate, bromide and phosphate concentrations remained below the calibration range (<4 µg/L, <0.4 µg/L, <0.2 µg/L and <4.4 µg/L, respectively). Formate concentration was determined to be above 160 µg/L.
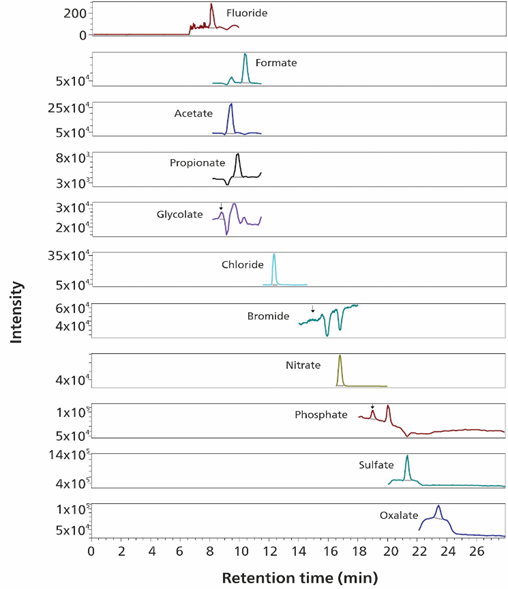
Figure 2. Fluoride (m/z 39), glycolate (m/z 75), acetate (m/z 59), propionate (m/z 73), formate (m/z 45), chloride (m/z 35), bromide (m/z 79), nitrate (m/z 62), phosphate (m/z 79), sulfate (m/z 97) and oxalate (m/z 89) (selected ion records) analysis is shown in in 1 mL ethanol. Separation was performed with a Metrosep A Supp 7-250/4.0 column (eluent: sodium carbonate/acetonitrile, high pressure gradient, flow rate0.8 mL/minute, column temperature 45°C). The software used was Waters EmpowerTM CDS.
Figure 3 shows some trace level impurities detected in NMP, methanol, 2-propanol, ethanol, and hydrogen peroxide (30%) samples. Automatic matrix elimination ensures that the same method can be used for various organic solvents and reactive solutions such as hydrogen peroxide.
Organic Acids and Inorganic Anions in Organic Matrices
To minimize matrix interactions and extend the life of analytical columns, organic matrices must be subjected to sample preparation prior to analysis.
MISP provides a way to automate sample preparation for many of these situations. Dosino units are ideal tools for precise aspiration and dosing, allowing them to perform all liquid handling steps, allowing users to perform other important laboratory tasks. In the application example, an appropriate volume of a special sample was injected into the pre-concentration column (PCC) and then the sample matrix was removed using the Inline Matrix Elimination (MiPCT-ME) technique.
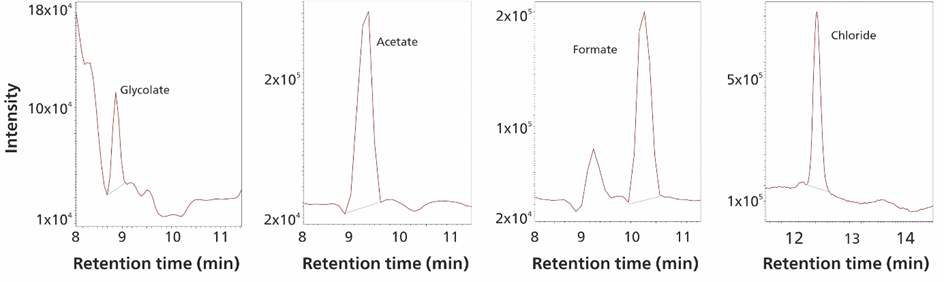
Figure 3. From left to right: Determination of glycolate (<0.4 μg/L) in 1-methyl-2-pyrrolidinone (NMP), acetate in ethanol (>166 μg/L), formate in 2-propanol (<1.8 μg/L), and chloride (>10 μg/L) in hydrogen peroxide (30%) peaks are shown. Sample volume was 1 mL. Selected ion recording (SIR) tracks were recorded with Waters EmpowerTM CDS.
Inorganic anions (fluoride, chloride, bromide, nitrate, nitrite, phosphate, sulfate, triflate (trifluoromethanesulfonate), tetrafluoroborate, hexafluorophosphate, and bistriflimide), as well as anions of organic acids (glycolate, acetate, formate, propionate, adipate, succinate, and sebacate) were analyzed with a Metrosep A Supp 7 - 250/4.0 column using a high-pressure binary gradient. Parallel detection of suppressed conductivity and selected m/z signals offers maximum precision of the analysis results (Table 1).
Agilent OpenLab CDS software was used to operate both the Metrohm system and the single quadrupole mass spectrometer (Agilent InfinityLab LC/MSD with API-Electrospray source). A feasibility study showed that these organic acids could be detected at trace levels at 5 µg/L. The study of the adipate ion is shown as an example in Figure 4.
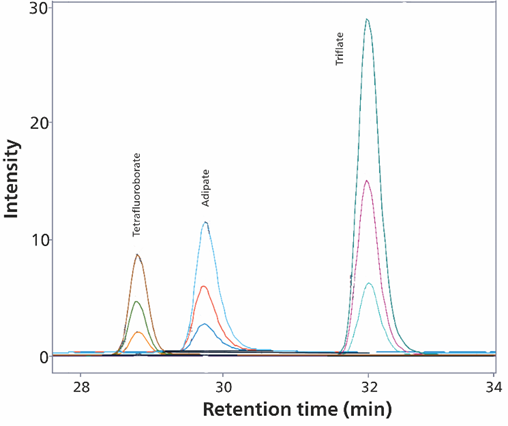
Figure 4. Selected ion monitoring of incoming adipate elution between tetrafluoroborate and trifluoromethanesulfonate (triflate) at three different concentration levels (10 µg/L, 25 µg/L and 100 µg/L) with Metrosep A Supp 7 - 250/4.0 column (eluent – gradient: sodium carbonate/acetonitrile, flow rate 0.8 mL/minute, column temperature 55 °C, sample volume 200 µL) is shown. The software used was Agilent OpenLab CDS.
Table 1. The m/z signal and retention times determined on the Metrosep A Supp 7-250/4.0 column are listed for each of the seven organic acids and eleven inorganic anions. (eluent-gradient: sodium carbonate/acetonitrile, flow rate 0.8 mL/minute, column temperature 55 °C).
|
Analyte |
m/z |
Retention time [min] |
|
Fluoride |
19 |
14.1 |
|
Glycolate |
75 |
15.6 |
|
Acetate |
59 |
16.5 |
|
Propionate |
73 |
17.9 |
|
Formate |
45 |
18.1 |
|
Chloride |
35 |
20.9 |
|
Nitrite |
46 |
22.1 |
|
Bromide |
79 |
23.7 |
|
Nitrate |
62 |
24.6 |
|
Phosphate |
79 |
28.0 |
|
Tetrafluoroborate |
87 |
28.7 |
|
Sulfate |
97 |
28.8 |
|
Succinate |
117 |
29.4 |
|
Adipate |
145 |
29.8 |
|
Triflate |
149 |
31.9 |
|
Sebacate |
201 |
33.6 |
|
Hexafluorophosphate |
145 |
47.4 |
|
Bistriflimide |
280 |
67.2 |
A summary of 18 components were analyzed in a 200 µL proprietary sample and their quantification limits were found to be in the range of 5 µg/L. In addition, three different concentrations of hexafluorophosphate (Figure 5) and bistriflimide (Figure 6) are shown to prove the low levels measurable with the IC-MS configuration described previously.
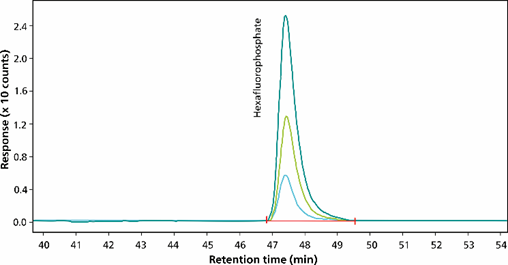
Figure 5. Hexafluorophosphate (m/z 145), measured by electrospray selected ion monitoring (ES-SIM) at concentrations of 10 µg/L, 25 µg/L and 50 µg/L, was separated using the Metrosep A Supp 7 - 250/4.0 column (eluent-gradient: sodium carbonate/acetonitrile, flow rate 0.8 mL/minute, column oven temperature 55 °C, sample volume 200 µL). The software used was Agilent OpenLab CDS.
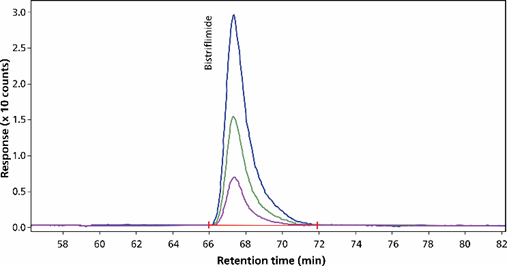
Figure 6. Bistriflimide (bis(trifluoromethane)-sulfonimide, m/z 280), measured at concentrations of 10 µg/L, 25 µg/L and 50 µg/L, was separated using a Metrosep A Supp 7 - 250/4.0 column and in negative mode detected by ion monitoring (eluent-gradient: sodium carbonate/ acetonitrile, flow rate 0.8 mL/minute, column temperature 55 °C, sample volume 200 µL). The software used was Agilent OpenLab CDS.
Table 2 shows that chloride, bromide, nitrite, nitrate, phosphate, and sulfate concentrations between 1-50 µg/L were detected with the applied configuration. The amount of fluoride was determined down to 250 μg/L. Fluoride's mass-to-charge ratio (m/z) of 19 explains that it is a relatively small molecule, which explains its lower signal intensity compared to the other ions listed.
Table 2. Mass signals (SIM in negative mode in m/z) and detection limits of seven inorganic anions are listed. Detection limits were determined by signal-to-noise ratio evaluation. Deprotonated phosphate and sulfate have the same mass-to-charge ratio; however, they are well separated by ion chromatography.
|
Analyte |
m/z |
Limits of detection (LOD) |
|
|
Concent. (μg/L) – 20 μL injection |
Amount (ng) |
||
|
Fluoride |
19 |
250 |
5 |
|
Chloride |
35 |
10 |
0.2 |
|
Nitrite |
46 |
50 |
1 |
|
Bromide |
79 |
5 |
0.1 |
|
Nitrate |
62 |
1 |
0.02 |
|
Phosphate |
97 |
10 |
0.2 |
|
Sulfate |
97 |
25 |
0.5 |
B. QUALITY CONTROL OF BEVERAGES
Organic Acids in Soft Drinks
The analysis of organic acids in soft drinks is of interest because they affect flavor and mouthfeel. While citric, malic, and tartaric acids are frequently used as acidifying agents in foods, acetic acid has been shown to have antimicrobial effects. Lactic acid can be used as an antimicrobial agent in food processes, for curing and pickling, to adjust pH or as a flavoring agent. In general, there is no limit on the acceptable daily intake for organic acids according to the Food and Agriculture Organization of the United Nations (FAO); however, the organic acid profile in food and beverages can be used as an indicator to monitor the degradation of product content. IC-MS is a suitable analytical method for the quantification of organic acids such as citric, shikimic, malic, quinic, tartaric and fumaric acids in food matrices.
Based on seven different soft drinks, a rapid and robust IC-MS method was developed for the quantification of the above-mentioned organic acids as well as inorganic anions (Table 3). Within a short run time (32 min), this method sensitively detects the co-elution of organic acids with MS (Figure 7). Thanks to its high capacity and symmetrical peaks, Metrosep A Supp 19 is an ideal separation column for samples with complex matrices found in the food and beverage industry.
Automatic calibration was done with Metrohm intelligent Partial Loop Injection Technique (MiPT) and the drawn quadratic lines are also compatible with the MS technique. Most calibrations cover the concentration range of 4–200 μg/L. Analytes with lower sensitivity (fluoride, shikimate, formate, propionate, nitrite, and fumarate) were measured down to 10 or 20 μg/L. Correlation coefficients (R2) for calibration were found to be at an acceptable level (0.997– 0.999). In this application example, chloride, nitrate, sulfate, and citrate ions were detected in all soft drinks analyzed. Nitrite, bromide, glutarate and adipate were not detected in any of the samples.
The easy-to-use IC-MS method for the determination of organic acids in apple spritzer, flavored mineral water, soda, and other soft drinks showed sufficient selectivity and sensitivity to detect co-elution compounds.
Table 3. List of the mass-charge (m/z) signal and retention times for 17 organic acids and seven inorganic anions analyzed in a single run by IC-MS.
|
Analyte |
m/z |
Retention time [min] |
|
Quinate |
191 |
5.1 |
|
Fluoride |
39 |
5.2 |
|
Shikimate |
173 |
5.5 |
|
Glycolate |
75 |
5.7 |
|
Lactate |
89 |
5.7 |
|
Acetate |
59 |
6.1 |
|
Propionate |
73 |
6.3 |
|
Formate |
45 |
6.5 |
|
Pyruvate |
87 |
6.7 |
|
Chloride |
35 |
8.0 |
|
Nitrite |
46 |
9.8 |
|
Bromide |
79 |
11.0 |
|
Nitrate |
62 |
12.4 |
|
Phosphate |
79 |
15.3 |
|
Sulfate |
97 |
16.7 |
|
Malate |
133 |
17.2 |
|
Malonate |
103 |
17.4 |
|
Glutarate |
131 |
17.8 |
|
Tartrate |
149 |
17.9 |
|
Succinate |
117 |
18.2 |
|
Adipate |
145 |
18.1 |
|
Oxalate |
89 |
18.9 |
|
Fumarate |
115 |
22.2 |
|
Citrate |
191 |
27.8 |
Oxyhalide Impurities in Bottled Water
Suppliers disinfect their water with chlorine, chlorine dioxide, chloramine, and ozone to provide clean drinking water. Toxic disinfection byproducts (DBPs) can be created by these disinfectants in combination with some naturally occurring substances that are already in water. For example, ozonation can convert naturally occurring bromide in the water supply to bromate, a potential carcinogen.
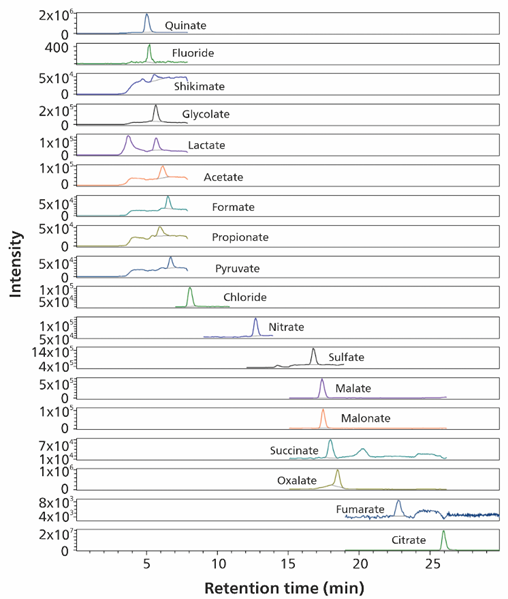
Figure 7. Quinate (3.98 mg/L), fluoride (0.35 mg/L), shikimate (0.18 mg/L), glycolate (1.11 mg/L), lactate (0.29 mg/L), acetate (0.52 mg/L), formate (0.69 mg/L), propionate (0.96 mg/L), pyruvate (0.22 mg/L), nitrate (0.12 mg/L), sulfate (3.43 mg/L), malate (1.34 mg/L), malonate (0.18 mg/L), succinate (0.05 mg /L) and fumarate (1.13 mg/L) analysis are shown in ice-tea (diluted 1:20). Nitrite, bromide, phosphate, glutarate, tartrate and adipate were below the calibration range. Chloride, oxalate, and citrate remained above the calibration range. Separation was performed on a Metrosep A Supp 19 - 150/4.0 column (eluent A: 8 mmol/L sodium carbonate, 0.25 mmol/L sodium hydrogen carbonate and 10% (v/v) methanol; eluent B: 80 mmol/L L sodium carbonate, 2.5 mmol/L sodium hydrogen carbonate and 10% (v/v) methanol; flow rate 0.75 mL/minute, column temperature 60 °C). Sample volume 10 μL. The software used was Waters EmpowerTM CDS.
Chlorite and chlorate can be formed through the chloramine refining process and may pose some health risks. According to the US Environmental Protection Agency (US EPA), limits in drinking water are set at 10 μg/L for bromate and 1 mg/L for chlorite. Bottled natural mineral and spring waters disinfected by ozonation are allowed to contain only 3 µg/L of bromate. The limit value of chlorate in drinking water is limited to 0.7 mg/L by the World Health Organization (WHO), although it is not currently regulated in the USA.
Ion chromatography (IC) and conductivity detection technique is the most preferred method to monitor and detect oxyhalides in water treatment processes. Thanks to the sufficient peak resolution of high-capacity anion exchange columns, analytes can be efficiently separated from sample matrix components and each other. However, the capabilities of the conductivity detection technique may be limited when it comes to the separation and sensitive detection of high concentrations (mg/L) of inorganic anions as well as trace levels (μg/L) of oxyhalides. Mass spectrometry (MS) as an additional (secondary) detection technique makes this analysis more powerful. With additional MS detection, sensitivity for oxyhalides is increased, making peak identification with selective mass signals easier and more accurate. Even analyte peaks with poor resolution can be easily quantified by mass signals (when they have different m/z values). Chromatogram times can be reduced to the point where there is minimal resolution between peaks, thus increasing sample throughput with the same or better quantification quality using IC-MS.
For drinking water quality control, it is important to detect low levels of the previously mentioned harmful oxyanions, as well as other common anions that may occur naturally in higher concentrations. In addition to conventional inorganic anions (fluoride, chloride, bromide, nitrate, phosphate, sulfate) in tap water, separation on a microbore 2 mm Metrosep A Supp 7 column enables quick measurement (12 minutes) of oxyhalides (bromate, chlorite, chlorate) at ng/L levels (Figure 8). The low flow rate required for the 2 mm IC column allows the conductivity detector to be directly connected to the MS without any flow splitter. Since adequate sensitivity levels for sensitive oxyanions are achieved using IC-MS, there is no need to add additional post-column solution. Thus, the setup remains easy and robust. In matrix-loaded samples, internal standards such as bromate with added 18O-chemical tracer can be used to achieve even higher sensitivity.
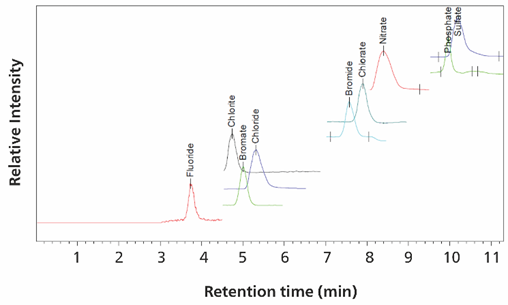
Figure 8. Image shows m/z levels in ultrapure water of 5 µg/L chlorite, 5 µg/L chlorate, 5 µg/L bromate, 100 µg/L bromide, 100 µg/L fluoride, 100 µg/L phosphate, 5 mg/L chloride, 5 mg/L nitrate and 5 mg/L sulfate peaks were created by overlaying. Separation was performed with a Metrosep A Supp 7 - 150/2.0 column (eluent-gradient: NaCO3/acetonitrile, flow rate 0.25 mL/minute, column temperature 45°C). Sample volume - 40 µL. The software was Waters EmpowerTM CDS.
An additional benefit of this method, especially for routine laboratories, is the use of a low volume sampling technique (Metrohm intelligent Pick-up Technique, MiPuT). Precisely the necessary quantity of sample is transported and injected into the IC. The injection volume can be selected individually for each sample. Additionally, thanks to the use of different injection volumes, automatic calibration can be made from a single stock standard. Therefore, not only low sample volumes can be handled and measured within short run time, but also the time usually needed for standard preparation is minimized, making the method more efficient and attractive for routine and high throughput analysis.
Using MiPuT for oxyhalides, calibration lines were established from 15 pg to 0.5 ng (absolute amounts) by injecting different volumes of a single standard solution (Figure 9). Limits of quantification (LOQ) were set in the ng/L range (e.g. 25 ng/L bromate with 100 μL sample volume; 2.5 pg bromate, Figure 10). Repeatability and spike studies (Table 4, Figure 11) show the method performance for the analysis of water samples.
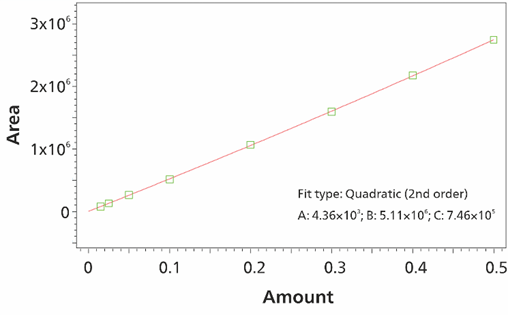
Figure 9. By injecting 3–100 µL of the stock standard solution (5 µg/L bromate) with MiPuT, a linear calibration line was created for bromate in the range of 15 pg to 0.5 ng. The software used was Waters EmpowerTM CDS.
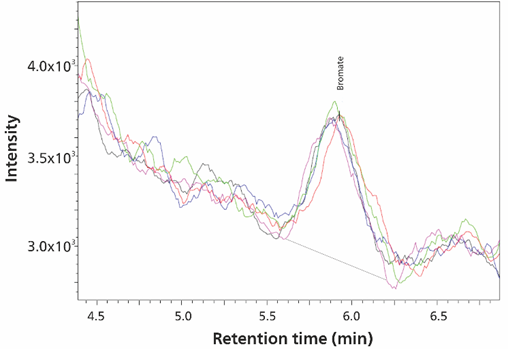
Figure 10. Detection of 25 ng/L bromate (2.5 pg bromate) with a sample volume of 100 µL is shown. The image created by overlay the analyzes shows the selected ion peak of five replicate analyses.
Table 4. Reproducibility study performed with a 50 µL control standard consisting of 0.5 µg/L chlorite, 0.5 µg/L chlorate, 0.5 µg/L bromate, 10 µg/L bromide and 10 µg/L phosphate.
|
Analyte |
Number of injections |
Area |
Height RSD [%] |
|
Chlorite |
12 |
25 |
8 |
|
Chlorate |
12 |
8 |
5 |
|
Bromate |
12 |
2 |
3 |
|
Bromide |
6 |
6 |
5 |
|
Phosphate |
12 |
5 |
3 |
In addition to bromate, chlorite and chlorate, the detection of perchlorate, as described in the EPA 332.0 standard, is also very important for the quality control of drinking water. Perchlorate is a contaminant that prevents iodine from being taken up by the thyroid, which can cause brain damage in newborns and infants. To learn more about the subject, you can review the IC-MS [5] and IC-MS/MS [6] application notes from Agilent.
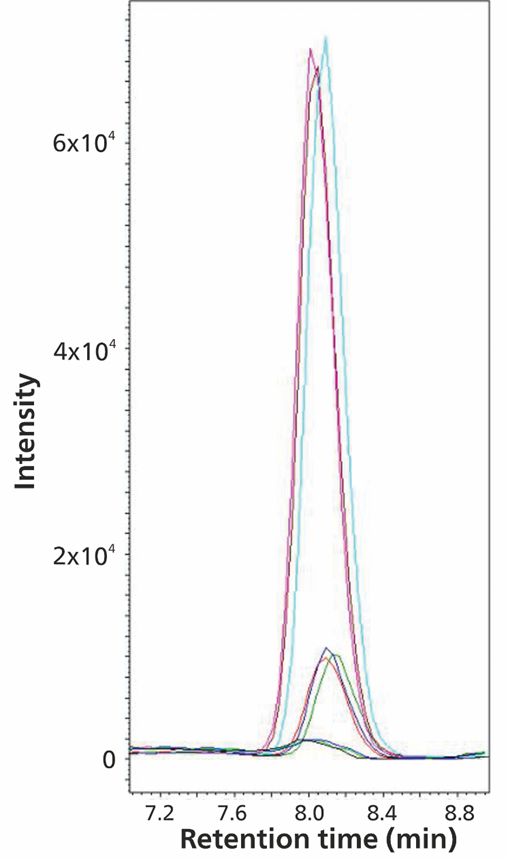
Figure 11. Chlorate spiked into tap water containing approximately 5 mg/L sulfate, with approximately 10 mg/L chloride and nitrate (spike level 1:1 µg/L and 2: 5 µg/L). The software used was Waters EmpowerTM CDS.
Combining the detection of trace levels of iodine species with the analysis of oxyhalides and inorganic anions once again demonstrates the power of the IC-MS combination. Iodide is an important micronutrient and is used in the synthesis of thyroid hormones. In the oxidative drinking water treatment process, iodide is oxidized to iodate, preventing the formation of potentially toxic disinfection byproducts. The Metrosep A Supp 7 column enables the separation of iodide and iodate as well as oxyhalides and inorganic anions in a single injection. Automated sample preparation (e.g. Inline Ultrafiltration) reduces manual workload and allows robust analysis of samples such as surface and drinking water, even if they contain particles. Quantification in the method was successfully carried out up to 0.25 µg/L for bromate and perchlorate, 0.5 µg/L for bromide, iodate, and iodide (Figure 12), and up to 5 µg/L for chlorite and chlorate.
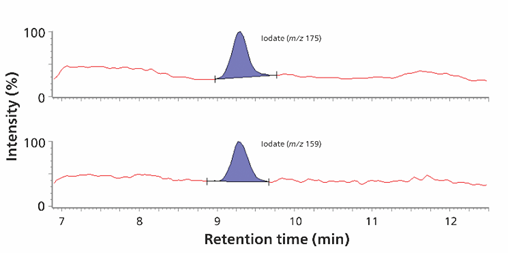
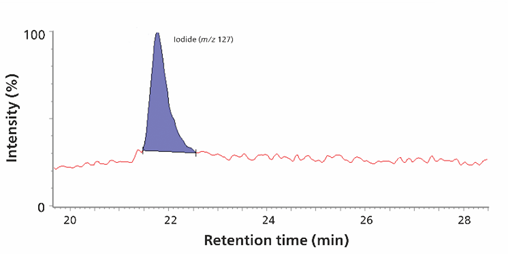
Figure 12. Identification of iodate (0.5 µg/L) as m/z 175 and m/z 159 (upper panel) and iodide (0.5 µg/L) as m/z 127 (lower panel) is shown. Analytes were separated on a Metrosep A Supp 7 - 250/4.0 column (eluent: 1.2 mmol/L sodium carbonate, 40% (v/v) acetonitrile, flow rate 0.8 mL/minute, column temperature 45°C). Sample volume is 100 µL. The software used were MagIC Net and Waters MassLynx™.
It requires sensitive analysis of oxyhalides and inorganic anions not only in water quality laboratories but also in forensic investigations. For more information, please review Metrohm Application Note AN-M-002.
C. MEDICAL APPLICATION: ORGANIC ACIDS IN URINE
Determination of organic acid levels in body fluids is important to help diagnose many diseases. Organic acids are among the parameters regularly measured in blood plasma or urine in hospitals. For example, the oxalate level in the urine may indicate hyperoxaluria. Glyceric acid is measured in pediatric patients to screen for inborn errors of amino acid metabolism. This rare disorder of D-glyceric aciduria is caused by mutations in the gene encoding the enzyme D-glycerate kinase. The resulting D-glycerate kinase deficiency can affect various metabolic pathways, leading to neurological symptoms and D-glyceric acid excretion.
In the application example below, glycerate, glycolate, oxalate and citrate were determined in the urine sample (Figure 13). Thanks to the high peak resolution power offered by the Metrosep A Supp 19 column, interference caused by chloride or other matrix components is minimized. Inorganic anions such as chloride and sulfate can also be analyzed in the same run. Isocratic elution with a single high-pressure pump ensures simple setup, robust method performance and easy handling. This makes the system setup simpler as there is no need to add other chemicals after the column.
Samples were sterile filtered, diluted 10-fold with boric acid (2% v/v) and injected into the IC using the Metrohm intelligent Partial Loop Injection Technique (MiPT). After separation on the Metrosep A Supp 19 column, analytes were detected by conductivity and MS techniques within 23 minutes (Figure 13). Organic acids were well separated from highly concentrated (e.g. chloride) inorganic anions, allowing precise measurement (Figure 14).
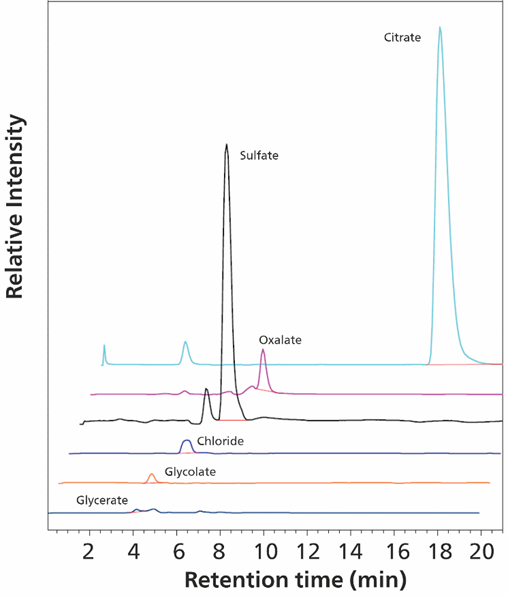
Figure 13. Mass signals of 180 ng glycolate (1.8 mg/L; 2 nmol), 860 ng oxalate (8.6 mg/L; 10 nmol) and 28.7 μg citrate (287 mg/L; 150 nmol) ions in the urine sample (diluted 10 times) are shown. Glycerate could not be detected. Separation was performed on a Metrosep A Supp 19 - 150/4.0 column (eluent: 30 mmol/L sodium carbonate, 1 mmol/L sodium hydrogen carbonate and 10% (v/v) methanol; flow rate 0.75 mL/minute, column temperature 60 °C). Sample volume 100 μL. The software used was Waters EmpowerTM CDS.
The calibration is plotted in the mg/L range (2–100 mg/L glycerate, 1–50 mg/L glycolate, 5–250 mg/L oxalate, and 100–5000 mg/L citrate) and the correlation coefficients (R2) were obtained as >0.999 for glycerate and glycolate and >0.98 for oxalate and citrate (MS signal, quadratic curve passing through 0). Anions chloride and sulfate were calibrated using conductivity signals because they showed a more linear correlation than the MS signal. Correlation coefficients (R2) in the range 10–500 mg/L are >0.9999 for both chloride and sulfate (conductivity, linear fit).
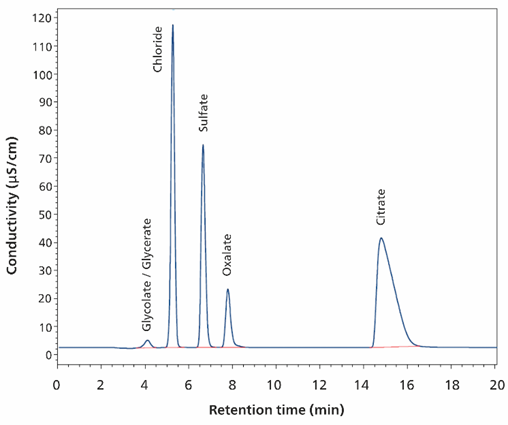
Figure 14. The conductivity signal of a standard containing 400 ng glycerate (2 mg/L; 3.8 nmol), 200 ng glycolate (1 mg/L; 2.7 nmol), 1 μg oxalate (5 mg/L; 11.2 nmol), 20 μg citrate (100 mg/L; 104.7 nmol), 2 μg chloride (10 mg/L; 57.1 nmol), and 2 μg sulfate (10 mg/L; 20.6 nmol) is shown. Separation was performed with a Metrosep A Supp 19 - 150/4.0 column (eluent: 30 mmol/L sodium carbonate, 1 mmol/L sodium hydrogen carbonate and 10% (v/v) methanol; flow rate 0.75 mL/minute, column temperature 60 °C). Sample volume 100 μL. The software used was Waters EmpowerTM CDS.
Conclusion
The liquid chromatography mass spectrometry (LC-MS) technique is a well-established analytical technique that has a diverse range of applications. When it comes to ionic analytes and/or matrices with high salt content, IC-MS is a powerful method that can be preferred. The highlights of the IC systems offered by Metrohm are listed below.
− Robust suppression (suppressor) technique
− An inert metal-free flow path
− Automated Inline Sample Preparation (MISP) and intelligent Injection (MiIT) Technique
− Various and reliable ion exchange columns with excellent separation power
IC-MS combines and enhances the benefits gained from the unique techniques of ion chromatography and mass spectrometry. It enables sensitive and selective determination of multiple anions, including organic acids (e.g. acetate or formate), as well as small inorganic anions (e.g. chloride and bromate) in one run.
In addition, MISP techniques automate sample preparation, making even complex matrices suitable for analysis with sensitive high-end detectors and increasing the efficiency of the method. MISP not only reduces human errors and manual processes, but also helps you save time and money, especially in laboratories with high analysis throughput.
References
1. Metrohm White Paper “Measuring organic acids and inorganic anions with ion chromatography mass spectrometry”
2. Metrohm White Paper “Coupling of IC and plasma mass spectrometry”
3. Metrohm White Paper “An introduction to ion chromatography mass spectrometry (IC-MS)”
4. Metrohm Application Note “Chlorite, chlorate, and perchlorate in explosion residue using IC/MS coupling”
5. Mathew, J.; Gandhi, J.; Hedrick, J. The Analysis of Perchlorate by Ion Chromatography/Mass Spectrometry. Agilent Technologies, Inc., 5989-0816EN 2004.
6. Mathew, J.; Gandhi, J.; Mohsin, S.; et al. Quantitative Analysis of Perchlorate by Ion Chromatography MS/MS. Agilent Technologies, Inc., 5989-7907EN 2008.
Author: Elif Metin Kulaksız - Metrohm Turkey Ion Chromatography Product Group Manager