Indispensable for the Leather Industry: Chrome Tanning
Raw hides and skins mostly obtained from cattle and sheep are a by-product/waste of meat and meat products industry, and they can easily rot and deteriorate if not protected under appropriate conditions.
Leather processing industry, in which businesses known as tanneries operate converts this natural material through a series of sequential and complex chemical and mechanical processes into a leather used for a wide variety of purposes.
So, the leather does not rot, does not deteriorate, does not stink, retains its shape, has a soft, flexible, strong and stable structure. Essentially, the whole process is the transformation of the protein known as collagen, which forms the fibrillary matrix of the raw hides/skins into a stronger, more durable and more stable state.
Collagen is the most abundant protein in animals. This fibrous, structural protein consists of molecules formed by the intertwining of three amino acid chains. This helix structure forms microfibrils; microfibrils fibrils; and fibrils collagen fibers.
Collagen has different types depending on the order of the chains formed by the combination of amino acids in its structure. Type I collagen consists of two α-1 and one α-2 chains. This strongest collagen constitutes 90% of the collagen in the human body as well as in animals.
The skin of the animal, which protects it from external factors and microorganisms in its health, can be structurally destroyed by the effect of microorganisms after the death of the animal or by the autolysis of cell enzymes in the skin itself.
At the most advanced stage, the raw hide can be broken down to the amino acids that make up the collagen protein. Raw hides/skins, which has a shrinkage temperature of around 65oC, can easily shrink with the effect of hot water, by breaking the hydrogen bonds that keep the hides as a whole.
In further stages, the skin structure gels and may even turn into glue. With the removal of water, which constitutes 60-65% of the raw leather, the leather dries and gains a hard, brittle structure, and when it is placed in water, it swells by absorbing water.
While it has such a form, the lifespan and area of raw leather is very limited, it can even
be said that it is useless. Due to the pollution released during the tanning of the leather, the leather industry is under strict pressure by environmental institutions and organizations.
Animal hides and skins can cause various diseases and a bad odor when left in the environment. The leather industry both has prevented them and converted raw hides into a usable, high value-added material, so carries out an environmental action.
In addition, since it is a labour-intensive industry, it also contributes to employment. As a result, as long as human beings continue to eat meat to meet their animal protein needs,
animal husbandry will continue and therefore hides/skins will be obtained as a by-product.

The process of transforming raw leather into finished leather is a long-term process brought about by technological knowledge and passion for leather. Leather manufacturing, first, starts with beamhouse operations for purpose of removing soluble proteins, lipids and
fats, hair or wool that are the components in the natural structure of the raw skin/hides from collagen fibres and bundles.
So, it is provided thus isolating the collagen network structure, changing and improving the active groups on the collagen protein suitable for bonding. The main purpose of these process steps, known as soaking, dehairing, liming, deliming, bating and degreasing, is to
purify the raw hides and make them ready for the tanning process.
The tanning is a the best step and designed according to the characteristics of the tanning material to be used. Because the tanning material used gives the leather a unique character. It is important in the selection of the tanning material that the leather to be produced will be used to meet the needs of the people.
It is important in the selection of the tanning material how the leather to be produced will be used to meet the needs of the people. Of course, the influence of fashion cannot be ignored at this stage. Tanning materials are classified according to their source.
Mineral tanning agents such as chrome, aluminum, zirconium, titanium, iron; vegetable tanning agents obtained from different parts of plants such as kebraco, mimosa, chestnut, pine, acorn, tara, gambir.
Also aldehyde, polymer, oil and synthetic (syntans) based tanning agents are widely used in the leather industry either alone or as well as used in combination with each other. On the other hand, the post-tanning processes applied after the neutralization step acquiring to leather by retanning, lubrication, dyeing, and finishing processes of elements such as handle, color, softness, flexibility, waterproofing, durability, strength, surface touch, homogeneity.
These procees should make leather suitable for the properties of the leather to be used used to manufacture the leather products. During these processes, the skin is also made into a flat layer by mechanical processes. The leather processing process is schematized in the figure below.

According to 2020 data, if around 23 billion square feet of leather is produced in the world today and the value of leather products in the global market reaches approximately 400 billion USD, the tanning industry’s awareness of the tanning power of chrome and the advantages it provides in the second half of the 1800s played a major role.
This was an important and impressive invention for the leather industry. Until this date, tannins of plants obtained from subtropical and tropical regions of the world were used in tanning. Vegetable tanning had a production process that took months.
However, the advent of chromium considerably shortened the process of converting raw hides to finished leather. On top of that, leathers tanned with this tanning agent were softer, more flexible, stronger, easier to dye and had higher hydrothermal resistance.
This has increased the interest in chromium so much that chromium is the main tanning
agent in 90% of the leathers produced in the world today. Chromium, which means color in Greek (atomic number 24; atomic weight 51,996), is also an important element in that Türkiye is the country with the richest chrome ore in the world after the Republic of South Africa.
Because chromium is very hard and has a melting point of 1857°C, it is used to provide hardness to metals and to make armored vehicles. The most important area of use is the production of stainless steel, where it is used together with nickel.
Thus, with the chromium oxide layer it forms, it covers the surface of the steel like
a film layer and provides resistance to corrosion. Chromium is found in nature as trivalent and therefore it can be used in powder form for tanning leathers.
Chromium (Cr) is one of the redox active heavy metals. It exists in trivalent chromium (Cr(III)) and hexavalent (Cr(VI)) forms in aqueous environments. Hexavalent chromium exists in various anionic forms such as chromate (CrO4 2−), hydro chromate (HCrO4
−), or dichromate (Cr2O7 2−), depending on the pH of the aqueous solution.
In wastewater, the permissible concentrations of trivalent and hexavalent chromium are 5 and 0.1 mg. At micro levels, Cr(III) is an essential trace element required in the human body for its physiological functions. It also plays a role in glucose, fat and protein metabolism by enhancing the effect of insulin.
Nutritionists have proven that chromium (III) content in the range of 13.4 mg/1,000 kcal and 8.4 - 23.7 mg/1,000 kcal on average is optimal for daily diets, but higher values are detrimental to human health. Even excess Cr(III) can cause a mutagenic effect.
Although it is not easy for Cr(III) complexes to pass through cell membranes, their accumulation around cells in the body causes cell surface morphological changes. Second, it causes cell membrane lipid injuries through disruption of cellular functions and integrity, eventually causing DNA damage.
Chrome tanned leather is known for its versatility, excellent hydrothermal stability, better dyeability and softness. However, chrome tanning is considered the most polluting process worldwide as it releases trivalent chromium ions [Cr(III)] in water bodies. However, compared to chrome-free tanning methods, chrome tanning is more suitable for the production of various leathers.
Chromium-tanned leathers interact better with retanning and lubricating chemicals.
However, Cr(III) in chrome tanning wastewater can be easily converted to Cr(VI) due to the presence of oxidizing agents such as dissolved oxygen and MnO2.
Also, pH fluctuations in a wastewater treatment plant can accelerate the oxidation of Cr(III) to Cr(VI). In addition, it has been determined that stored leather and other leather products deteriorate over time due to the effects of humidity, temperature and UV rays.
During this process, lubricants, tanning agents and auxiliary substances, replace the
molecules in the leather structure and then free radicals are formed. Consequently, these radicals become potential drivers of Cr(III) oxidation to Cr(VI).
Leather products have been proven to contain Cr(VI) content over time, making it difficult to guarantee that the Cr(III) used in leather tanning is harmless to consumers of leather products.
Hexavalent chromium [Cr(VI)] threatens terrestrial and marine life due to its high toxicity. In addition, Cr(VI) is carcinogenic, mutagenic and allergenic to humans and the death of
organisms.
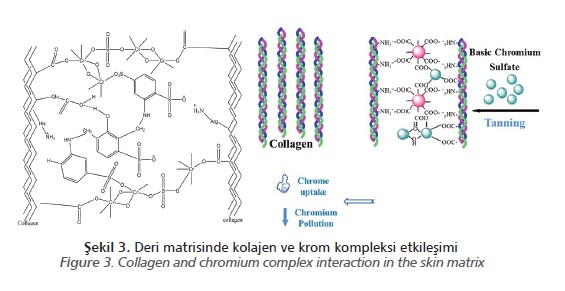
Only 55-70% of all chromium salts that enter the tanning liquor during tanning are bound
to the leather and the remainder passes into the wastewater. The main part of the chrome waste water comes from the tanning bath and a significant amount is likewise discharged from the washing and retanning processes.
Chromium concentrations in spent liquor range from 2000 mg/l to 5000 mg/l, while environmental regulatory agencies worldwide allow less than 20 mg/l of total chromium for discharge of wastewater into public sewers.
Practically, it is possible to reduce chromium concentrations in the tannery waste stream to allowable levels, but this is highly capital-intensive, which imposes a burden of operating
costs on the tanneries. This challenge requires appropriate technologies to minimize or eliminate chromium in wastewater for the sustainability of the leather industry.
Chrome tanning technology remains an environmental issue as it causes global concern. In terms of sustainability and to have a clean leather industry, chromium pollution should be reduced. For this, there are intensive studies on chromium-free tanning methods, that is, alternative tanning methods to chromium, but a method that can provide this in all aspects has not been found yet.
Other reduction methods are reducing the use of chromium, reusing tanning baths and recovering chromium from waste baths. Apart from environmental pollution, chromium also has significant negative effects on human health.
Tannery workers can be exposed to chromium by breathing or contact. Especially +6 valent chromium can cause carcinogenic and even fatal effects. Therefore, serious measures should be taken in terms of occupational health and safety and employees should be encouraged to comply with these measures.
Literature
1) Zhang C, Lin J, Jia X, Peng B., (2016). A salt-free and chromium discharge minimizing tanning technology: The novel cleaner integrated chrome tanning process. Journal of Cleaner Production.; 112(1):1055-1063. Doi: doi.org/10.1016/j.jclepro.2015.07.155
2) Uddin,, M. M., Hasan, M. J., Mahmud,, Y., Tuj-Zohra, F., & Ahmed, S. (2020). Evaluating Suitability of Glutaraldehyde Tanning in Conformity with Physical Properties of Conventional Chrome-Tanned Leather. Textile & Leather Review, 3(3), 135–145.
Doi: https://doi.org//10.31881/tlr.2020.09
3) Wang L, Chen M, Li J, Jin Y, Zhang Y, Wang Y. A novel substitution-based method for effective leaching of chromium (III) from chromium-tanned leather waste: The thermodynamics, kinetics and mechanism studies. Waste Managment. 2020 Feb 15, 103:276-284. Doi: https://doi.org//10.1016/j.wasman.2019.12.039
4) Muralidharan, V., Palanivel, S., Balamaran, M., 2022, Turning problem into possibility: A comprehensive review on leather solidwaste into-valorization attemps for leather processing, Journal of Cleaner Production, 367 Doi: https://doi.org/10.1016/j.clepro.2022.133021
5) Kanagaraj, G., Babu, N.C., Mandal, A. 2008, Recovery and reuse of chromium from chrome tanning waste water aiming towards zero discharge of pollution. J. Clean.Prod.16, 1807-1813 Doi: https://doi.org/10.1016/j.jclepro.2007.12.005
6) Anderson, R.A., 1981. Nutritional role of chromium. Sci. Total Environ. 17, 13e29.
https://doi.org/10.1016/0048-9697(81)90104-2.
7) Oruka, R.O., Selvarajan, R., Ogola, H.J.O., Edokpayi, J.N., 2020, Contemporary and future direction of chromium tanning and management in sub Saharan Africa tanneries. Process Safety and Environmental Protection.369-386 Doi: https://doi.org/10.1016/j.psep.2019.11.013
Asst. Prof. Fazlı Akyüz
Department Of Textiles-Clothing, Footwear and Leather,
Leather Technology Program
İstanbul University Vocational School of Technical Sciences

 The process of transforming raw leather into finished leather is a long-term process brought about by technological knowledge and passion for leather. Leather manufacturing, first, starts with beamhouse operations for purpose of removing soluble proteins, lipids and
fats, hair or wool that are the components in the natural structure of the raw skin/hides from collagen fibres and bundles.
So, it is provided thus isolating the collagen network structure, changing and improving the active groups on the collagen protein suitable for bonding. The main purpose of these process steps, known as soaking, dehairing, liming, deliming, bating and degreasing, is to
purify the raw hides and make them ready for the tanning process.
The tanning is a the best step and designed according to the characteristics of the tanning material to be used. Because the tanning material used gives the leather a unique character. It is important in the selection of the tanning material that the leather to be produced will be used to meet the needs of the people.
It is important in the selection of the tanning material how the leather to be produced will be used to meet the needs of the people. Of course, the influence of fashion cannot be ignored at this stage. Tanning materials are classified according to their source.
Mineral tanning agents such as chrome, aluminum, zirconium, titanium, iron; vegetable tanning agents obtained from different parts of plants such as kebraco, mimosa, chestnut, pine, acorn, tara, gambir.
Also aldehyde, polymer, oil and synthetic (syntans) based tanning agents are widely used in the leather industry either alone or as well as used in combination with each other. On the other hand, the post-tanning processes applied after the neutralization step acquiring to leather by retanning, lubrication, dyeing, and finishing processes of elements such as handle, color, softness, flexibility, waterproofing, durability, strength, surface touch, homogeneity.
These procees should make leather suitable for the properties of the leather to be used used to manufacture the leather products. During these processes, the skin is also made into a flat layer by mechanical processes. The leather processing process is schematized in the figure below.
The process of transforming raw leather into finished leather is a long-term process brought about by technological knowledge and passion for leather. Leather manufacturing, first, starts with beamhouse operations for purpose of removing soluble proteins, lipids and
fats, hair or wool that are the components in the natural structure of the raw skin/hides from collagen fibres and bundles.
So, it is provided thus isolating the collagen network structure, changing and improving the active groups on the collagen protein suitable for bonding. The main purpose of these process steps, known as soaking, dehairing, liming, deliming, bating and degreasing, is to
purify the raw hides and make them ready for the tanning process.
The tanning is a the best step and designed according to the characteristics of the tanning material to be used. Because the tanning material used gives the leather a unique character. It is important in the selection of the tanning material that the leather to be produced will be used to meet the needs of the people.
It is important in the selection of the tanning material how the leather to be produced will be used to meet the needs of the people. Of course, the influence of fashion cannot be ignored at this stage. Tanning materials are classified according to their source.
Mineral tanning agents such as chrome, aluminum, zirconium, titanium, iron; vegetable tanning agents obtained from different parts of plants such as kebraco, mimosa, chestnut, pine, acorn, tara, gambir.
Also aldehyde, polymer, oil and synthetic (syntans) based tanning agents are widely used in the leather industry either alone or as well as used in combination with each other. On the other hand, the post-tanning processes applied after the neutralization step acquiring to leather by retanning, lubrication, dyeing, and finishing processes of elements such as handle, color, softness, flexibility, waterproofing, durability, strength, surface touch, homogeneity.
These procees should make leather suitable for the properties of the leather to be used used to manufacture the leather products. During these processes, the skin is also made into a flat layer by mechanical processes. The leather processing process is schematized in the figure below.
 According to 2020 data, if around 23 billion square feet of leather is produced in the world today and the value of leather products in the global market reaches approximately 400 billion USD, the tanning industry’s awareness of the tanning power of chrome and the advantages it provides in the second half of the 1800s played a major role.
This was an important and impressive invention for the leather industry. Until this date, tannins of plants obtained from subtropical and tropical regions of the world were used in tanning. Vegetable tanning had a production process that took months.
However, the advent of chromium considerably shortened the process of converting raw hides to finished leather. On top of that, leathers tanned with this tanning agent were softer, more flexible, stronger, easier to dye and had higher hydrothermal resistance.
This has increased the interest in chromium so much that chromium is the main tanning
agent in 90% of the leathers produced in the world today. Chromium, which means color in Greek (atomic number 24; atomic weight 51,996), is also an important element in that Türkiye is the country with the richest chrome ore in the world after the Republic of South Africa.
Because chromium is very hard and has a melting point of 1857°C, it is used to provide hardness to metals and to make armored vehicles. The most important area of use is the production of stainless steel, where it is used together with nickel.
Thus, with the chromium oxide layer it forms, it covers the surface of the steel like
a film layer and provides resistance to corrosion. Chromium is found in nature as trivalent and therefore it can be used in powder form for tanning leathers.
Chromium (Cr) is one of the redox active heavy metals. It exists in trivalent chromium (Cr(III)) and hexavalent (Cr(VI)) forms in aqueous environments. Hexavalent chromium exists in various anionic forms such as chromate (CrO4 2−), hydro chromate (HCrO4
−), or dichromate (Cr2O7 2−), depending on the pH of the aqueous solution.
In wastewater, the permissible concentrations of trivalent and hexavalent chromium are 5 and 0.1 mg. At micro levels, Cr(III) is an essential trace element required in the human body for its physiological functions. It also plays a role in glucose, fat and protein metabolism by enhancing the effect of insulin.
Nutritionists have proven that chromium (III) content in the range of 13.4 mg/1,000 kcal and 8.4 - 23.7 mg/1,000 kcal on average is optimal for daily diets, but higher values are detrimental to human health. Even excess Cr(III) can cause a mutagenic effect.
Although it is not easy for Cr(III) complexes to pass through cell membranes, their accumulation around cells in the body causes cell surface morphological changes. Second, it causes cell membrane lipid injuries through disruption of cellular functions and integrity, eventually causing DNA damage.
Chrome tanned leather is known for its versatility, excellent hydrothermal stability, better dyeability and softness. However, chrome tanning is considered the most polluting process worldwide as it releases trivalent chromium ions [Cr(III)] in water bodies. However, compared to chrome-free tanning methods, chrome tanning is more suitable for the production of various leathers.
Chromium-tanned leathers interact better with retanning and lubricating chemicals.
However, Cr(III) in chrome tanning wastewater can be easily converted to Cr(VI) due to the presence of oxidizing agents such as dissolved oxygen and MnO2.
Also, pH fluctuations in a wastewater treatment plant can accelerate the oxidation of Cr(III) to Cr(VI). In addition, it has been determined that stored leather and other leather products deteriorate over time due to the effects of humidity, temperature and UV rays.
During this process, lubricants, tanning agents and auxiliary substances, replace the
molecules in the leather structure and then free radicals are formed. Consequently, these radicals become potential drivers of Cr(III) oxidation to Cr(VI).
Leather products have been proven to contain Cr(VI) content over time, making it difficult to guarantee that the Cr(III) used in leather tanning is harmless to consumers of leather products.
Hexavalent chromium [Cr(VI)] threatens terrestrial and marine life due to its high toxicity. In addition, Cr(VI) is carcinogenic, mutagenic and allergenic to humans and the death of
organisms.
According to 2020 data, if around 23 billion square feet of leather is produced in the world today and the value of leather products in the global market reaches approximately 400 billion USD, the tanning industry’s awareness of the tanning power of chrome and the advantages it provides in the second half of the 1800s played a major role.
This was an important and impressive invention for the leather industry. Until this date, tannins of plants obtained from subtropical and tropical regions of the world were used in tanning. Vegetable tanning had a production process that took months.
However, the advent of chromium considerably shortened the process of converting raw hides to finished leather. On top of that, leathers tanned with this tanning agent were softer, more flexible, stronger, easier to dye and had higher hydrothermal resistance.
This has increased the interest in chromium so much that chromium is the main tanning
agent in 90% of the leathers produced in the world today. Chromium, which means color in Greek (atomic number 24; atomic weight 51,996), is also an important element in that Türkiye is the country with the richest chrome ore in the world after the Republic of South Africa.
Because chromium is very hard and has a melting point of 1857°C, it is used to provide hardness to metals and to make armored vehicles. The most important area of use is the production of stainless steel, where it is used together with nickel.
Thus, with the chromium oxide layer it forms, it covers the surface of the steel like
a film layer and provides resistance to corrosion. Chromium is found in nature as trivalent and therefore it can be used in powder form for tanning leathers.
Chromium (Cr) is one of the redox active heavy metals. It exists in trivalent chromium (Cr(III)) and hexavalent (Cr(VI)) forms in aqueous environments. Hexavalent chromium exists in various anionic forms such as chromate (CrO4 2−), hydro chromate (HCrO4
−), or dichromate (Cr2O7 2−), depending on the pH of the aqueous solution.
In wastewater, the permissible concentrations of trivalent and hexavalent chromium are 5 and 0.1 mg. At micro levels, Cr(III) is an essential trace element required in the human body for its physiological functions. It also plays a role in glucose, fat and protein metabolism by enhancing the effect of insulin.
Nutritionists have proven that chromium (III) content in the range of 13.4 mg/1,000 kcal and 8.4 - 23.7 mg/1,000 kcal on average is optimal for daily diets, but higher values are detrimental to human health. Even excess Cr(III) can cause a mutagenic effect.
Although it is not easy for Cr(III) complexes to pass through cell membranes, their accumulation around cells in the body causes cell surface morphological changes. Second, it causes cell membrane lipid injuries through disruption of cellular functions and integrity, eventually causing DNA damage.
Chrome tanned leather is known for its versatility, excellent hydrothermal stability, better dyeability and softness. However, chrome tanning is considered the most polluting process worldwide as it releases trivalent chromium ions [Cr(III)] in water bodies. However, compared to chrome-free tanning methods, chrome tanning is more suitable for the production of various leathers.
Chromium-tanned leathers interact better with retanning and lubricating chemicals.
However, Cr(III) in chrome tanning wastewater can be easily converted to Cr(VI) due to the presence of oxidizing agents such as dissolved oxygen and MnO2.
Also, pH fluctuations in a wastewater treatment plant can accelerate the oxidation of Cr(III) to Cr(VI). In addition, it has been determined that stored leather and other leather products deteriorate over time due to the effects of humidity, temperature and UV rays.
During this process, lubricants, tanning agents and auxiliary substances, replace the
molecules in the leather structure and then free radicals are formed. Consequently, these radicals become potential drivers of Cr(III) oxidation to Cr(VI).
Leather products have been proven to contain Cr(VI) content over time, making it difficult to guarantee that the Cr(III) used in leather tanning is harmless to consumers of leather products.
Hexavalent chromium [Cr(VI)] threatens terrestrial and marine life due to its high toxicity. In addition, Cr(VI) is carcinogenic, mutagenic and allergenic to humans and the death of
organisms.
 Only 55-70% of all chromium salts that enter the tanning liquor during tanning are bound
to the leather and the remainder passes into the wastewater. The main part of the chrome waste water comes from the tanning bath and a significant amount is likewise discharged from the washing and retanning processes.
Chromium concentrations in spent liquor range from 2000 mg/l to 5000 mg/l, while environmental regulatory agencies worldwide allow less than 20 mg/l of total chromium for discharge of wastewater into public sewers.
Practically, it is possible to reduce chromium concentrations in the tannery waste stream to allowable levels, but this is highly capital-intensive, which imposes a burden of operating
costs on the tanneries. This challenge requires appropriate technologies to minimize or eliminate chromium in wastewater for the sustainability of the leather industry.
Chrome tanning technology remains an environmental issue as it causes global concern. In terms of sustainability and to have a clean leather industry, chromium pollution should be reduced. For this, there are intensive studies on chromium-free tanning methods, that is, alternative tanning methods to chromium, but a method that can provide this in all aspects has not been found yet.
Other reduction methods are reducing the use of chromium, reusing tanning baths and recovering chromium from waste baths. Apart from environmental pollution, chromium also has significant negative effects on human health.
Tannery workers can be exposed to chromium by breathing or contact. Especially +6 valent chromium can cause carcinogenic and even fatal effects. Therefore, serious measures should be taken in terms of occupational health and safety and employees should be encouraged to comply with these measures.
Literature
1) Zhang C, Lin J, Jia X, Peng B., (2016). A salt-free and chromium discharge minimizing tanning technology: The novel cleaner integrated chrome tanning process. Journal of Cleaner Production.; 112(1):1055-1063. Doi: doi.org/10.1016/j.jclepro.2015.07.155
2) Uddin,, M. M., Hasan, M. J., Mahmud,, Y., Tuj-Zohra, F., & Ahmed, S. (2020). Evaluating Suitability of Glutaraldehyde Tanning in Conformity with Physical Properties of Conventional Chrome-Tanned Leather. Textile & Leather Review, 3(3), 135–145.
Doi: https://doi.org//10.31881/tlr.2020.09
3) Wang L, Chen M, Li J, Jin Y, Zhang Y, Wang Y. A novel substitution-based method for effective leaching of chromium (III) from chromium-tanned leather waste: The thermodynamics, kinetics and mechanism studies. Waste Managment. 2020 Feb 15, 103:276-284. Doi: https://doi.org//10.1016/j.wasman.2019.12.039
4) Muralidharan, V., Palanivel, S., Balamaran, M., 2022, Turning problem into possibility: A comprehensive review on leather solidwaste into-valorization attemps for leather processing, Journal of Cleaner Production, 367 Doi: https://doi.org/10.1016/j.clepro.2022.133021
5) Kanagaraj, G., Babu, N.C., Mandal, A. 2008, Recovery and reuse of chromium from chrome tanning waste water aiming towards zero discharge of pollution. J. Clean.Prod.16, 1807-1813 Doi: https://doi.org/10.1016/j.jclepro.2007.12.005
6) Anderson, R.A., 1981. Nutritional role of chromium. Sci. Total Environ. 17, 13e29.
https://doi.org/10.1016/0048-9697(81)90104-2.
7) Oruka, R.O., Selvarajan, R., Ogola, H.J.O., Edokpayi, J.N., 2020, Contemporary and future direction of chromium tanning and management in sub Saharan Africa tanneries. Process Safety and Environmental Protection.369-386 Doi: https://doi.org/10.1016/j.psep.2019.11.013
Asst. Prof. Fazlı Akyüz
Department Of Textiles-Clothing, Footwear and Leather,
Leather Technology Program
İstanbul University Vocational School of Technical Sciences
Only 55-70% of all chromium salts that enter the tanning liquor during tanning are bound
to the leather and the remainder passes into the wastewater. The main part of the chrome waste water comes from the tanning bath and a significant amount is likewise discharged from the washing and retanning processes.
Chromium concentrations in spent liquor range from 2000 mg/l to 5000 mg/l, while environmental regulatory agencies worldwide allow less than 20 mg/l of total chromium for discharge of wastewater into public sewers.
Practically, it is possible to reduce chromium concentrations in the tannery waste stream to allowable levels, but this is highly capital-intensive, which imposes a burden of operating
costs on the tanneries. This challenge requires appropriate technologies to minimize or eliminate chromium in wastewater for the sustainability of the leather industry.
Chrome tanning technology remains an environmental issue as it causes global concern. In terms of sustainability and to have a clean leather industry, chromium pollution should be reduced. For this, there are intensive studies on chromium-free tanning methods, that is, alternative tanning methods to chromium, but a method that can provide this in all aspects has not been found yet.
Other reduction methods are reducing the use of chromium, reusing tanning baths and recovering chromium from waste baths. Apart from environmental pollution, chromium also has significant negative effects on human health.
Tannery workers can be exposed to chromium by breathing or contact. Especially +6 valent chromium can cause carcinogenic and even fatal effects. Therefore, serious measures should be taken in terms of occupational health and safety and employees should be encouraged to comply with these measures.
Literature
1) Zhang C, Lin J, Jia X, Peng B., (2016). A salt-free and chromium discharge minimizing tanning technology: The novel cleaner integrated chrome tanning process. Journal of Cleaner Production.; 112(1):1055-1063. Doi: doi.org/10.1016/j.jclepro.2015.07.155
2) Uddin,, M. M., Hasan, M. J., Mahmud,, Y., Tuj-Zohra, F., & Ahmed, S. (2020). Evaluating Suitability of Glutaraldehyde Tanning in Conformity with Physical Properties of Conventional Chrome-Tanned Leather. Textile & Leather Review, 3(3), 135–145.
Doi: https://doi.org//10.31881/tlr.2020.09
3) Wang L, Chen M, Li J, Jin Y, Zhang Y, Wang Y. A novel substitution-based method for effective leaching of chromium (III) from chromium-tanned leather waste: The thermodynamics, kinetics and mechanism studies. Waste Managment. 2020 Feb 15, 103:276-284. Doi: https://doi.org//10.1016/j.wasman.2019.12.039
4) Muralidharan, V., Palanivel, S., Balamaran, M., 2022, Turning problem into possibility: A comprehensive review on leather solidwaste into-valorization attemps for leather processing, Journal of Cleaner Production, 367 Doi: https://doi.org/10.1016/j.clepro.2022.133021
5) Kanagaraj, G., Babu, N.C., Mandal, A. 2008, Recovery and reuse of chromium from chrome tanning waste water aiming towards zero discharge of pollution. J. Clean.Prod.16, 1807-1813 Doi: https://doi.org/10.1016/j.jclepro.2007.12.005
6) Anderson, R.A., 1981. Nutritional role of chromium. Sci. Total Environ. 17, 13e29.
https://doi.org/10.1016/0048-9697(81)90104-2.
7) Oruka, R.O., Selvarajan, R., Ogola, H.J.O., Edokpayi, J.N., 2020, Contemporary and future direction of chromium tanning and management in sub Saharan Africa tanneries. Process Safety and Environmental Protection.369-386 Doi: https://doi.org/10.1016/j.psep.2019.11.013
Asst. Prof. Fazlı Akyüz
Department Of Textiles-Clothing, Footwear and Leather,
Leather Technology Program
İstanbul University Vocational School of Technical Sciences