Chlorinated solvents have been used effectively in a variety of applications over many years. Producers today supports the following applications [1].
• Urethane foam blowing,
• Coatings and adhesives formulation,
• Paint formulation and stripping,
• Aerosol products formulation (only perchlorethylene and methylene chloride for certain applications outside the EU),
• Metal cleaning/degreasing,
• Dry cleaning,
• Chemical processing industry,
• Pharmaceuticals manufacturing.
The popularity of these solvents in these industries derived from their low flammability and reactivity, ease of evaporation, and strong dissolving power [2]. Volatile organic compounds (VOCs) are major air pollutants that must be controlled under increasingly stringent environmental regulations.
Among them, chlorinated VOCs have been widely used in the chemical industry including the manufacture of herbicides, plastics, and solvents.
Uses outside the chemical industry include solvent degreasing in the automotive and aerospace industries, dry cleaning solvents in the garment industries, and solvent cleaning in the electronic industries [3].
Volatile organic compounds (VOCs) are the source of serious environmental issues and their emission has been subjected to more and more stringent legislation [4].
Essentially all substances, both natural and man-made, are toxic to some degree. Toxicity is defined as the ability of a substance to produce any harmful effect to a living organism, at some level or frequency of exposure, by inhalation, ingestion, or direct skin or eye contact.
Risk is determined by exposure and hazard. Thus, the potential risk of a hazardous substance can be considerably reduced with proper handling, such as the use of engineering controls, fume hoods, respirators, chemical goggles and other safety equipment, all of which limit exposure. Table 1. The chemical and physical properties of MC, TCE and PCE
Table 1. The chemical and physical properties of MC, TCE and PCE
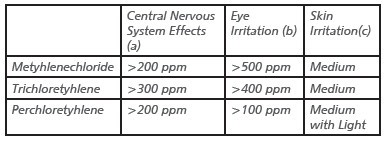
Table 2. Summary of Potential Health Effects in Humans from Acute Exposure to Chlorinated Solvents
(a) For example: headache, dizziness, sleepiness. Usually these effects are reversible if the person is removed from the area and given fresh air.
(b) May cause pain, wash with pure/disttilled water without the use of soap.
(c) Effects become more severe on covered skin (under clothing, gloves) and upon prolonged or repeated exposure. All chlorinated solvents can cause defatting of the skin with repeated exposure [1].
The health problem most frequently faced in industries handling large quantities of organic solvents is the necessity for their workers to be continuously exposed, day after day, to an atmosphere containing small proportions of solvent vapors.
Inhalation of sufficiently small amounts of solvent vapors need have no ill effect, since, as has been pointed out, they may be completely thrown off by the body. The amount of vapor which may be safely inhaled in this manner varies with the solvent [5].
There are commercial technologies available for chlorinated VOCs abatement. Among adsorption and separation technologies, activated carbon adsorption is widely used in industry because of easy operation, low operating cost, and efficient recovery of most chlorinated VOCs.
However, it has been recognized that activated carbon frequently encounters problems such as combustion, pore blocking. As a result, alternative adsorbents have received attention.
Hydrophobic zeolites have proven to be an advancement in controlling chlorinated VOCs [3]. Lee J. and oth. adsorption equilibria of seven chlorinated volatile organic compounds on mesoporous silicate MCM-48 were obtained using a gravimetric technique.
For comparison purposes, three organic sorbents (activated carbon, activated carbon fiber and nonionic polymer resin, SP850) and three inorganic sorbernts (DAY zeolite, mesoporous silicate MCM-41, and chromium-impregnated MCM-48) were used to investigate adsorption characteristics of trichloroethene chosen as a model compound of chlorinated volatile organic compounds. In this study it is aimed for the development of an energy efficient adsorption and catalytic destruction of chlorinated VOCs in MCM-48.
Absorption of VOCs into selective solvents constitutes an alternative technique to other processes for their removal (e.g., thermal or catalytic incineration, adsorption on activated carbon, biological, or membrane processes). Removal of chlorinated species by incineration can result in the formation of hydrochloric acid and dioxins.
In addition, the thermal regeneration of sorbents may be problematic and hinder the use of this technique. Scrubbing of waste gases using selective solvents therefore represents an alternative, hazardless route for a reversible process, and has been investigated in particular by German workers.
The reversible process allows both the cleaning of waste air and the possible recovery of the VOCs: this technique has been used for decades for other pollutants (e.g., sulfur dioxide) with a number of organic media… The selection of suitable solvents for absorption is generally made by considering the following criteria:
High absorption capacity, high selectivity with respect to other gases, and low toxicity and volatility. Whereas the formation of pollutant-solvent complex was demonstrated for sulfur dioxide in various solvents, it appears from previous investigations that the absorption of chlorinated VOCs should not result in the formation of such complexes.
However, interactions between VOC and solvent molecules result in possible affinity or in mutual repulsion and are to govern the absorption capacity of the solvent (2).

Figure 1. Detection frequencies of four solvents in groundwater by land use, at or above a concentration of 0.2 μg/L [9]
Chlorinated solvents have been widely used in a variety of industrial, military, and household applications since the 1940s. Their extensive use, improper handling and disposal practices, as well as accidental spills resulted in widespread subsurface and groundwater contamination.
Betts K showed that common chlorinated solvents including tetrachloroethene (PCE), trichloroethene (TCE), carbon tetrachloride (CT), dichloromethane (DCM) and 1,1,1trichloroethane (TCA)3 tend to form dense nonaqueous-phase liquids (DNAPLs), which move gravitationally along interconnected fractures and form pools in low points in fractured bedrock formations.
A significant mass of chlorinated solvents in a fractured rock site diffuses into low-permeability zones,4−6 and back-diffusion into water-bearing fractures serves as a longterm source of groundwater contamination [6].
As Simsir B. Stated, in situ measurements demonstrated that reductive dechlorination in the sediment attenuated chlorinated compounds before reaching the water column Microcosms established with creek sediment or in situ incubated Bio-Sep beads degraded C1−C3 chlorinated solvents to less-chlorinated or innocuous
products. the microbiological and hydrogeological characterization demonstrated that microbial processes at the fractured bedrock−sediment interface were crucial for preventing contaminants reaching the water column, emphasizing the relevance of this critical zone environment for contaminant attenuation [6].
According to the research of Betts K., the in situ redox manipulation technique creates a kind of a permeable reactive barrier, said Bob Puis, senior soil research chemist at EPA’s National Risk Management Research Laboratory in Ada, Okla.
As groundwater passes through the barrier, organic solvent contaminants in the groundwater are destroyed. Since the barriers were put in plarp there has been no detectable chromate found in either of the treated plumes.
The in situ permeable treatment barrier intercepts contaminants in groundwater and effectively prevents further spread of the targeted pollutants in the plume [7].
Helt B. et al. In order to reduce the environmental effects of chlorinated organic compounds, they have developed a method in aqueous environment and high temperature. They described for converting micromole quantities of chlorinated volatile organic compounds to CO2 and CH3Cl for C and Cl isotope ratio determinations.
This method provides an improved analytical approach for using C and Cl isotope ratios in studies of the biodegradation of chlorinated volatile organic compounds in the environment [8].
Chlorinated solvents are not very soluble in water, but they can still cause contamination of surface or groundwater. Additionally, because chlorinated solvents are heavier than water, large spills will tend to collect at low points, creating a concentrated source for continuing contamination.
Even process water that has come in contact with chlorinated solvents will contain some chlorinated solvent and should be handled as a hazardous waste stream. The main causes of groundwater and soil contamination are negligence and improper storage, handling and disposal.
Contaminated soil and water are difficult and costly to clean. Therefore, avoiding leaks and spills that can cause groundwater and soil contamination is imperative. Do not dispose of contaminated water in the sewer or septic system or pour it on the ground.
 Emine Akbulut
Chemical Engineer (M.Sc )
Sales & Marketing Specialist
Ef Kimya Tic. ve San. Ltd. Şti.
Emine Akbulut
Chemical Engineer (M.Sc )
Sales & Marketing Specialist
Ef Kimya Tic. ve San. Ltd. Şti.
References
[1] Chlorınated Solvents Product Stewardshıp Manual, Olin Chlorinated Organics
[2] Robert D. Morrison, Brian L. Murphy, and Richard E. Doherty, Chlorinated Solvents, Part 12, Page 260-272
[3] Lee J., Shim W., Suh S., 2003, Adsorption of Chlorinated Volatile Organic Compounds on MCM-48, American Chemical Society
[4] Hadjoudj R., Monnier H., 2204, Absorption of Chlorinated VOCs in High-Boiling Solvents: Determination of Henry’s Law Constants and Infinite Dilution Activity Coefficients, Ind. Eng. Chem. Res., Vol. 43, No. 9
[5] Lillian G., 1945, Using Organic Solvents Safely in Industry, Safety Research Institute, New York
[6] Şimşir B., Yan J., Im J., Graves D., 2017, Natural Attenuation in Streambed Sediment Receiving Chlorinated Solvents from Underlying Fracture Networks, Environ. Sci. Technol. 2017, 51, 4821−4830
[7] Betts K., 1998, Novel Barrier Remediates Chlorinated Solvents, American Chemical Society
[8] Holt B., Sturchio N., Abrajano N., 1997, Conversion of Chlorinated Volatile Organic Compounds to Carbon Dioxide and Methyl Chloride for Isotopic Analysis of Carbon and Chlorine, A n a l y t i c a l C h e m i s t r y , V o l . 6 9
[9] Moran M., Zogorski J., Squaillace, 2007, Chlorinated Solvents in Groundwater of the United States, Environmental Science Technology, Geological Survey, 1608 Mountain View Road, Rapid City, South Dakota 57702

 Table 1. The chemical and physical properties of MC, TCE and PCE
Table 1. The chemical and physical properties of MC, TCE and PCE


 Emine Akbulut
Chemical Engineer (M.Sc )
Sales & Marketing Specialist
Ef Kimya Tic. ve San. Ltd. Şti.
Emine Akbulut
Chemical Engineer (M.Sc )
Sales & Marketing Specialist
Ef Kimya Tic. ve San. Ltd. Şti.