Aluminium and Al alloys
The surface pre-treatment of aluminium and aluminium alloys includes cleaning, degreasing, pickling and possibly the creation of conversion layers. Aluminium castings are
not pickled, but blasted.
The equipment and processes for degreasing and pickling are basically the same as for the pretreatment of ferrous materials. By far the largest proportion of aluminium products are those that are to be anodised.
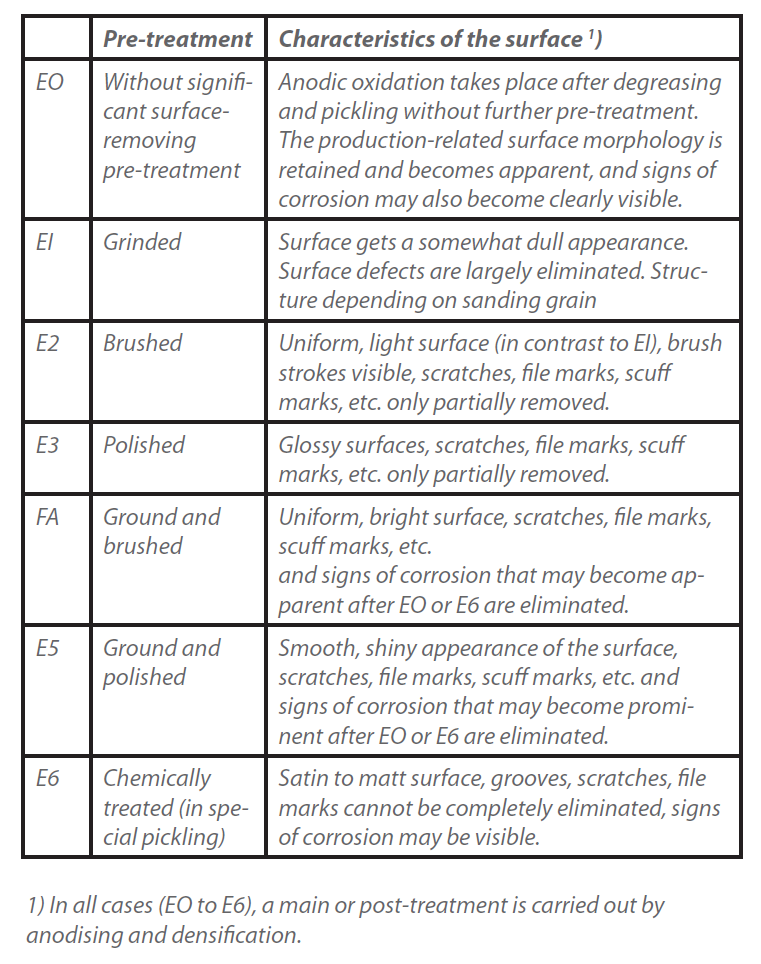
Their pre-treatment, in particular that of semi-finished aluminium products, is compiled in DIN 17611, an extract of which is given in Table 1.
Organic solvents, aqueous, emulsified or weakly alkaline degreasing solutions and acid degreasers with a low phosphating effect (1 to 10 % orthophosphoric acid, technically
85%) can be used to degrease aluminium surfaces. When degreasing with alkaline agents, rinse abundantly with water afterwards.
Pickling
Pickling is mostly alkaline, rarely acidic. For alkaline pickling, 10 to 30% sodium hydroxide or potassium hydroxide is used as a pickling agent. The pickling time is 1 to 3 min. The pickling is followed by thorough rinsing with water and neutralisation with nitric acid, 20%.
For acid pickling, sulphuric acid and/or hydrofluoric acid is used. Special protective measures are required when pickling with hydrofluoric acid.
Creation of Conversion Layers
Conversion coatings are applied with the aim of improving the adhesion of organic coatings to the aluminium surface, which is in itself smooth and dense.
Oxide, anodised and phosphate layers are of particular importance here, as they are often integrated into the process of organic coating. The process corresponds to the production of conversion coatings.
Copper
The top layer is removed from oxidised or tarnished copper surfaces after previous degreasing by processing with abrasive fleece rollers, blasting with a pumice/water mixture or by descaling in diluted H2S04. Additional pickling in Na2S207 is also possible.
Lacquers adhere poorly to smooth copper surfaces. Pretreatment includes degreasing with organic solvents and mechanical pre-treatment of the surface. In special cases, such as in printed circuit board technology, the adhesive strength of photoresists can be significantly increased by spraying with e.g. pumice powder.
Zinc
Especially for a subsequent organic coating, spray-galvanised and zinc die-castings do not require any special surface pre-treatment; zinc sheet, hot-dip galvanised components and electro-galvanised require pre-treatment, which consists in degreasing and phosphating the surface.
After a treatment period of up to 30 min, the surface is rinsed cold and hot and then dried. Older surfaces covered with a zinc carbonate layer are freed from dust and dirt by mechanical processes.
Pretreatment of plastics usually has two objectives. Firstly, degreasing to achieve appropriate wettability with the processes described above and secondly, roughening the surface to create mechanical adhesion centres.
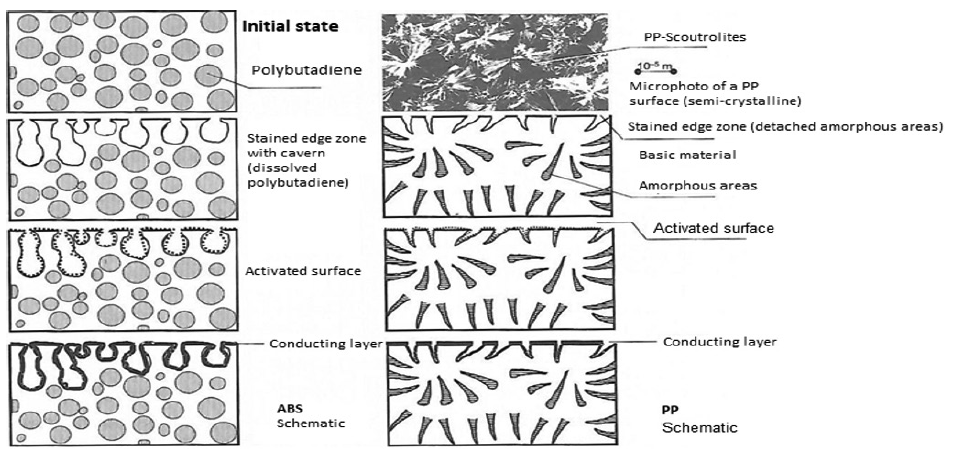
A typical example is the pretreatment of ABS plastics and polypropylene for metallisation without external current (see Fig. 1), where a chemical treatment is applied. For plastics that are difficult to attack chemically, plasma is suitable for cleaning and activation.
An extreme case is the pre-treatment of Teflon films (PTFE) with molten sodium. Foils treated in this way can be bonded and, for special components, metallised without external
current via activation.
Rinsing
In almost all active treatment steps in surface technology that are carried out in an aqueous medium, they are followed by rinsing. This fact is often not particularly emphasised in process descriptions. The aim of rinsing is:
• To stop the active process in a defined way,
• To minimise electrolyte carry-over and thus save bath ingredients,
• Prevent a possible chemical secondary reaction of the surface,
• As well as to enable recovery of ingredients and of the water.
If deionised water is used for rinsing (defined purity, water hardness) and suitable equipment and technologies are used, the required quality of the coating is achieved if the rinsing criterion R is met.
At the same time, the demand for water and wastewater savings from an environmental and economic point of view can be met.
Here Co is the concentration of salts in the electrolyte or the treatment solution and C is the equilibrium concentration in the rinsing water. Equilibrium is established between the
ions passing from the substrate surface into the rinse water and the ions returning to the surface.
This case applies to the non-flowing rinse water. This corresponds to the condition of
a standing sink. The reciprocal value of the rinsing criterion R indicates the degree of dilution X to be achieved in the rinse.
Compliance with the rinsing criterion R and the resulting limit value of the permissible concentration of salts in rinsing water residues on the fabric determine the fresh water quantity.
With an entrained electrolyte quantity of D in 1/min, the required fresh water quantity is W in 1/min:
W = D . R
In words: The amount of fresh water required to perform a rinsing step is influenced by the rinsing criterion R and the amount of electrolyte or solution D introduced into the rinsing
water.
The water requirement increases with the concentration of the entrained electrolyte and the area rinsed in the time unit.
Residues of the treatment solutions adhere to all fabric surfaces. Numerous test results are available on this. In general, one can say:
• Highly concentrated electrolytes (e.g. nickel) result in high drag-out losses, they amount to about 40 to 70 ml/ m2 for flat, vertical surfaces.
• With minimum drip times of 6 to 10 seconds, the dragout losses tend towards the minimum.
• By adding wetting agents it is possible to minimise the drag-out losses by approx. 20 to 30%.
For R, empirical values are used in practice to determine the fresh water requirement for flushing. Values for the flushing criterion are given in Table 2.
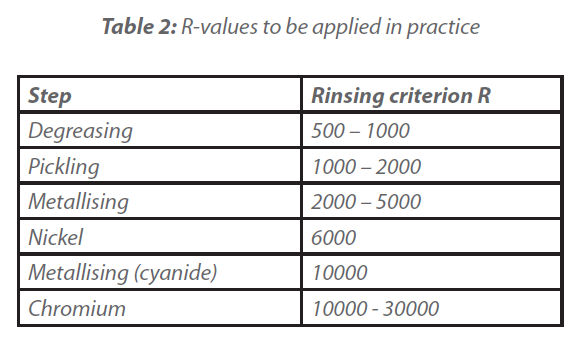
Rinsing criteria of 100 000 and more are required to achieve sophisticated end surfaces in the fields of optics, electronics, vacuum technology and Si technology. Depending on the requirements and conditions, rinsing can be carried out with fully demineralised water, drinking water or circulating or process water.
Technically, immersion rinsing is the easiest to realise, whereby the goods are immersed in a constant volume of rinsing water. The concentration of the introduced electrolyte increases with the number of “immersions”. This rinsing technique is called stand rinsing.
A constant influx of fresh water eliminates the disadvantages of stand rinsing at the expense of higher water consumption, flow rinsing. Single-stage rinsing, uncontrolled fresh water inflow and insufficient fabric movement increase the water requirement and worsen the rinsing result.
The obvious solution is to combine warewashing techniques, thus exploiting the advantages and minimising the disadvantages. This is realised, for example, by a multiple rinse cascade with a downstream flow rinse bath.
With cascade rinsing, the water from the previous rinsing tank is fed into the next one in a series of rinsing tanks.
The rinsing water and the workpieces to be rinsed move in counter-current, whereby the workpieces are rinsed last in the tank into which the fresh water is fed and first in the rinsing tank with the highest electrolyte concentration. These findings for rinsing practice are reflected in the results in Table 3.
[caption id="attachment_148389" align="aligncenter" width="696"]
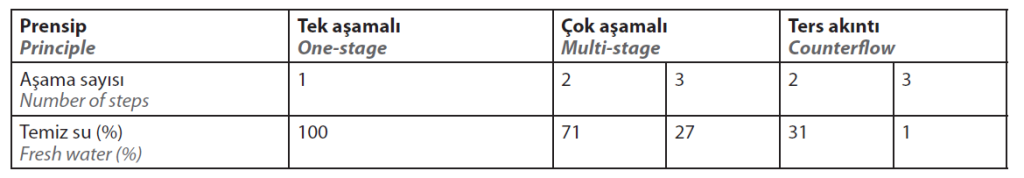
Table 3: Rinsing technology and fresh water demand[/caption]
Spray rinsing is particularly suitable for flat and smooth workpieces, such as printed circuit boards. The rinsing water is sprayed under pressure from spray nozzles onto the surface of the workpiece and thus a high enrichment of the electrolyte in the rinsing water can be achieved.
In principle, a system requires only one spray rinsing station, but this does not allow the rinsing water to be separated. In addition, simple measures are possible to a limited extent
to reduce bath carry-over into the rinse water. These are:
• Remove the electrolyte film by draining, squeezing, wiping off, blowing off, suctioning off, spinning off and shaking off,
• Drainage-friendly design (small smooth surfaces; no scooping or capillary action),
• Lower concentrated baths,
• Increased bath temperature (means lower viscosity),
• Reduced extension speeds.
Rinsing after the last coating step is followed by drying, with the aim of removing the water film very quickly and evenly from the fabric surface, e.g. to prevent tarnishing or staining.
A wetting agent is often added to the final rinse.
References:
• Vorbehandlung als Herausforderung, Metalloberflaeche, RITUPER
• Reinigung in die Fertigung integrieren, Metalloberflaeche, LANDAU
• Prozesssicherheit in der Galvanotechnik, Metalloberflaeche, PENZ
• Wirtschaftliche und umweltgerechte Teilereinigung, Galvanotechnik, FALLOT
İzzet Aydın
General Manager
Hillebrand Chemicals Kimyasal Pazarlama Ltd. Şti.

 Organic solvents, aqueous, emulsified or weakly alkaline degreasing solutions and acid degreasers with a low phosphating effect (1 to 10 % orthophosphoric acid, technically
85%) can be used to degrease aluminium surfaces. When degreasing with alkaline agents, rinse abundantly with water afterwards.
Organic solvents, aqueous, emulsified or weakly alkaline degreasing solutions and acid degreasers with a low phosphating effect (1 to 10 % orthophosphoric acid, technically
85%) can be used to degrease aluminium surfaces. When degreasing with alkaline agents, rinse abundantly with water afterwards.

 Rinsing criteria of 100 000 and more are required to achieve sophisticated end surfaces in the fields of optics, electronics, vacuum technology and Si technology. Depending on the requirements and conditions, rinsing can be carried out with fully demineralised water, drinking water or circulating or process water.
Technically, immersion rinsing is the easiest to realise, whereby the goods are immersed in a constant volume of rinsing water. The concentration of the introduced electrolyte increases with the number of “immersions”. This rinsing technique is called stand rinsing.
A constant influx of fresh water eliminates the disadvantages of stand rinsing at the expense of higher water consumption, flow rinsing. Single-stage rinsing, uncontrolled fresh water inflow and insufficient fabric movement increase the water requirement and worsen the rinsing result.
The obvious solution is to combine warewashing techniques, thus exploiting the advantages and minimising the disadvantages. This is realised, for example, by a multiple rinse cascade with a downstream flow rinse bath.
With cascade rinsing, the water from the previous rinsing tank is fed into the next one in a series of rinsing tanks.
The rinsing water and the workpieces to be rinsed move in counter-current, whereby the workpieces are rinsed last in the tank into which the fresh water is fed and first in the rinsing tank with the highest electrolyte concentration. These findings for rinsing practice are reflected in the results in Table 3.
[caption id="attachment_148389" align="aligncenter" width="696"]
Rinsing criteria of 100 000 and more are required to achieve sophisticated end surfaces in the fields of optics, electronics, vacuum technology and Si technology. Depending on the requirements and conditions, rinsing can be carried out with fully demineralised water, drinking water or circulating or process water.
Technically, immersion rinsing is the easiest to realise, whereby the goods are immersed in a constant volume of rinsing water. The concentration of the introduced electrolyte increases with the number of “immersions”. This rinsing technique is called stand rinsing.
A constant influx of fresh water eliminates the disadvantages of stand rinsing at the expense of higher water consumption, flow rinsing. Single-stage rinsing, uncontrolled fresh water inflow and insufficient fabric movement increase the water requirement and worsen the rinsing result.
The obvious solution is to combine warewashing techniques, thus exploiting the advantages and minimising the disadvantages. This is realised, for example, by a multiple rinse cascade with a downstream flow rinse bath.
With cascade rinsing, the water from the previous rinsing tank is fed into the next one in a series of rinsing tanks.
The rinsing water and the workpieces to be rinsed move in counter-current, whereby the workpieces are rinsed last in the tank into which the fresh water is fed and first in the rinsing tank with the highest electrolyte concentration. These findings for rinsing practice are reflected in the results in Table 3.
[caption id="attachment_148389" align="aligncenter" width="696"] Table 3: Rinsing technology and fresh water demand[/caption]
Spray rinsing is particularly suitable for flat and smooth workpieces, such as printed circuit boards. The rinsing water is sprayed under pressure from spray nozzles onto the surface of the workpiece and thus a high enrichment of the electrolyte in the rinsing water can be achieved.
In principle, a system requires only one spray rinsing station, but this does not allow the rinsing water to be separated. In addition, simple measures are possible to a limited extent
to reduce bath carry-over into the rinse water. These are:
• Remove the electrolyte film by draining, squeezing, wiping off, blowing off, suctioning off, spinning off and shaking off,
• Drainage-friendly design (small smooth surfaces; no scooping or capillary action),
• Lower concentrated baths,
• Increased bath temperature (means lower viscosity),
• Reduced extension speeds.
Rinsing after the last coating step is followed by drying, with the aim of removing the water film very quickly and evenly from the fabric surface, e.g. to prevent tarnishing or staining.
A wetting agent is often added to the final rinse.
References:
• Vorbehandlung als Herausforderung, Metalloberflaeche, RITUPER
• Reinigung in die Fertigung integrieren, Metalloberflaeche, LANDAU
• Prozesssicherheit in der Galvanotechnik, Metalloberflaeche, PENZ
• Wirtschaftliche und umweltgerechte Teilereinigung, Galvanotechnik, FALLOT
İzzet Aydın
General Manager
Hillebrand Chemicals Kimyasal Pazarlama Ltd. Şti.
Table 3: Rinsing technology and fresh water demand[/caption]
Spray rinsing is particularly suitable for flat and smooth workpieces, such as printed circuit boards. The rinsing water is sprayed under pressure from spray nozzles onto the surface of the workpiece and thus a high enrichment of the electrolyte in the rinsing water can be achieved.
In principle, a system requires only one spray rinsing station, but this does not allow the rinsing water to be separated. In addition, simple measures are possible to a limited extent
to reduce bath carry-over into the rinse water. These are:
• Remove the electrolyte film by draining, squeezing, wiping off, blowing off, suctioning off, spinning off and shaking off,
• Drainage-friendly design (small smooth surfaces; no scooping or capillary action),
• Lower concentrated baths,
• Increased bath temperature (means lower viscosity),
• Reduced extension speeds.
Rinsing after the last coating step is followed by drying, with the aim of removing the water film very quickly and evenly from the fabric surface, e.g. to prevent tarnishing or staining.
A wetting agent is often added to the final rinse.
References:
• Vorbehandlung als Herausforderung, Metalloberflaeche, RITUPER
• Reinigung in die Fertigung integrieren, Metalloberflaeche, LANDAU
• Prozesssicherheit in der Galvanotechnik, Metalloberflaeche, PENZ
• Wirtschaftliche und umweltgerechte Teilereinigung, Galvanotechnik, FALLOT
İzzet Aydın
General Manager
Hillebrand Chemicals Kimyasal Pazarlama Ltd. Şti.