New Retinol Alternative: Next Generation Sequencing Discovers Hydroxypinacolone Retinoate Efficacy
Summary
Retinol is the anti-aging ingredient with high efficacy in wrinkle reduction and skin renewal. In contrast to its benefits, retinol elicits side effects including inflammation, spongiosis, redness and dryness especially during long-term use and overdosing.
In the past, pinacolone ester retinoids were proposed and developed, but their clear mechanistic and efficacious advantage over the classical use of retinol remained unclear. To elucidate and compare their molecular mechanism and advantages, hydroxypinacolone
retinoate (HPR) – a well-known retinoid alternative of pinacolone esters was used in comparison in a whole gene expressional analysis by next generation sequencing and in a in vivo anti-wrinkle study.
These data reveal that HPR activates a similar subset of genes, but also improves gene expressional efficacy on gene sets that are involved in extracellular matrix regulation, cornified envelop, keratinization and protein digestion and absorption compared to retinol.
It’s in vivo efficacy on wrinkle reduction on hemi-face is significantly and to the same extend increased as in retinol treated skin and approves therewith as a convenient retinol replacer without the flaws.
Introduction
Retinoids are defined as Vitamin A derivatives based on beta-ionone. They are incorporated via nutrition and occur as Retinol and Retinyl esters of animal origin or as plant-based carotenoids. Retinoids have a plethora of functions depending on tissue and active form.
Specifically, their function in distinct tissues depends on the respective retinoid derivative and retinoic acid stereoisomers, e.g. vision functionality is facilitated by retinal binding rhodopsin in the retina cells, whereas tissue differentiation e.g. skin epidermis differentiation is regulated by retinoic acid (RA) stereoisomers binding to their nuclear receptors in the respective tissue and activating gene expression.
Here, 9-cis RA acts mainly as ligand of the retinoid-X receptors RXRs, whereas Retinoic acid receptors RAR bind 9-cis RA and all-trans RA (ATRA)
1; 2. As retinoids are hydrophobic, they need to be transported in the aqueous tissue environment by retinoid binding proteins which divide according to their appearance in plasma retinol-binding protein (RBP 4), the interstitial retinol-binding protein (RBP 3) and the cellular retinol-binding proteins (CRBPs or RBP 1, RBP2, RBP 5 and RBP 7).
These transport proteins not only facilitate the solubilization of retinoids, but also protect them from oxidation. Further, the cellular retinoic acid binding proteins (CRABP) mediate
the interaction between RA and its receptors RXR and RAR attributing CRABP co-activator properties.
When RAR receptors bind to RA this results in a conformational change allowing heterodimerization with other receptors such as RXRs, LXRs and PPARγ among others. This promiscuity of the RAR receptors mediates the pleiotropic effects of RAs in the various tissues.
For example, the heterodimer complex subunits are altered with the differentiation status of keratinocytes with RARα/RXRα dominating in the basal layer and RARγ/RXRα in the suprabasal layer. Beside its gene expression regulating function, RA exhibits non-transcriptional pathway activity via RAR and RXR activation distinct from there activity as transcription factors.
For instance, RARα forms a complex with G protein alpha Q (Gαq) coupled receptor in lipid
rafts in response to RA and activate p38 MAPK signaling
3 and it binds PI3K subunits RA dependently in order to activate AKT and subsequently ERK1/2
4.
As mentioned above retinoids affect differentiation processes, specifically they are essential for keratinocyte differentiation, where Keratin and heparin-binding epidermal growth factor gene expression thereby orchestrating skin differentiation and barrier function.
Explicitly KRT4 and KRT1
3 in the suprabasal layer is upregulated and KRT2 which occurs in the stratum spinosum is downregulated
5; 6. Further, cell division of basal keratinocytes is increased resulting in cell proliferation and subsequently epidermal thickening
5.
By means of this, Vitamin A deficiency could result in skin problems and retinoids are used in the treatment of keratinization disorders, e.g. epidermolysis bullosa simplex and epidermolytic ichthyosis, but also in the treatment of psoriasis and acne vulgaris
6.
During aging processes, keratinization and epidermal thickness is impaired. Here, retinoids
are widely used in topical formulations to decelerate skin phenotypes including wrinkles, roughness, laxity, sallowness, scaling and dryness.
Especially, UVinduced photoaging was reported to be improved by the use of retinoids
7. Mechanistically, wrinkles, laxity and roughness are mitigated via upregulation of Collagen I, III and IV ameliorating dermal-epidermal junctions and dermal tissue elasticity
8; 9.
Summarizing, retinoids have been proven to exhibit advantageous effects in the topical treatment of aging phenotypes but also of psoriasis and acne vulgaris. Even though, recent reports focus attention on their limitations and side effects.
One limitation of retinoids is their decreasing bioavailability in prolonged uses due to their increasing inactivation by CYP26 family enzymes and other cytochrome P450 members which are induced by retinoids and their RAR/RXR signaling therewith forming a negative feedback-loop
10.
By this effect, a vitamin A deficiency can occur resulting in unfortunate skin conditions. Further detrimental retinoid side effects are characterized as ‘retinoid reaction’ with burning sensation, pruritus and erythema occurring at the beginning of retinoid use.
This may be attributed to the activation of the irritation receptor TRPV1 by retinoids and declines after adaptation to their use
11. But also, customers and patients observed inflammation, peeling, erythema, and dried skin after long-term use of retinoids.
Concluding, all these observed adverse effects are manifold and their molecular cause is not clarified in detail. Thus, dermatologists and cosmetic industries perceived the necessity to develop retinoid alternatives with good efficacy but reduced adverse effects.
Here, Varani and colleagues developed pinacolonic ester-substituted retinoids with less irritant and adverse effects
12. Hydroxypinacolone retinoate (HPR) was the best derivative thereof transmitting the market. But until now, a detailed mechanistic comparison to elucidate its advantages over all-trans retinol was missing.
Therefore, in this study, we performed a whole transcriptomic sequencing (RNA-Seq) approach to elucidate and compare the molecular effects of a stabilized blend of HPR (in the following referred as “the ingredient”) vs. the benchmark all-trans retinol in an in vitro
3D model. Further, we could show in an in vivo study that the ingredient exhibits the same efficacy as alltrans retinol on wrinkle reduction.
Materials and Method
RNA Sequencing of 3D Reconstituted Epidermal Skin Models
EpiDerm reconstituted 3D models (MatTek, Bratislava, Slovakia) were cultivated for 72h. Then the investigational product SymRenewTM HPR 1 (INCI: Diisopropyl Adipate,
Hydroxypinacolone Retinoate, Tocopherol) and the benchmark all-trans retinol are daily recurring applied for 5 days.
This ingredient is a dedicated blend of Hydroxypinacolone retinoate facilitating a good stability. For each experimental condition, three biological replicates were analyzed. Epi-
Derm models are harvested in RNA lysis buffer.
RNA was isolated using Quiagen RNAeasy Mini kit (Quiagen, Hilden, Germany. Samples were processed for Illumina TruSeq, stranded, poly(A) enriched RNA library preparation. Sequencing was performed on Illumina NextSeq, v2.5 with 75bp and 30 Mio reads per sample.
RNA Sequencing Data Analysis
Following base calling, demultiplexing and trimming of Illumina adaptor residuals, data was mapped against the GRCh38 genome and FPKM for each gene was obtained. FPKM were averaged and normalized to either the untreated control or to the all-trans retinol treated sample.
Genes with a natural logarithmic fold change smaller than -1≤ and bigger than ≥1 and p-value < 0.05 (genes with FPKM < 3 were excluded) were considered to be significantly differentially expressed. Functional enrichment and clustering were performed using DAVID Bioinformatics Resources 6.8. Functional clusters with Bonferroni corrected p-value ≤ 0.05 were considered as significant.
In vivo Study on Wrinkle Reduction
This in vivo study was performed per determinations of Resolution 466/12 of the National Health Council for Regulatory Guidelines and Standards for Research Involving Humans including a written Informed Consent.
The 96 research subjects (mean age: 55 ± 5 years, Phototype (Fitzpatrick) 3% phototype II and 97% phototype III) were instructed to stop using any topical-use products on their face 48 hours before starting the study.
They also got instruction on the schedules for testing in laboratories and not to use any products during the study period. The study was based on the hypothesis that the treatment with the investigational products per directions can reduce the signs of aging of facial skin.
The methodology used in this study evaluates the reduction of wrinkles and facial expression lines by image analysis. The images of the faces were obtained at the baseline, after 14 and 28 days of in-home use.
The research subjects applied the investigational products twice a day, once in the morning and once at night. The research subjects were instructed to apply one investigational product on the right side of the face, and another investigational product on the left side of the face or to keep a side of the face (right or left) as a control.
The side (right or left) of application was randomized among the subjects. According to the morphology of the face of each research subject, size, and location of the region with the presence of wrinkles and expression lines, a region was determined for the analysis.
The images were obtained through the equipment Primos CR Large field (Canfield®) with the maximum resolution of 50 micrometers at the initial condition of the study and after 14 and 28 days of home use of the investigational products. The images obtained were analyzed using the VAM software, version 5.9.7, Canfield Scientific Inc, by inserting parallel lines over the determined region.
Statistical method was ANOVA, with Dunnet post-test, considering a 95% confidence interval. The intensity of the wrinkles and lines was determined using the values of the roughness parameter Rv %, that refers to valley of greater depth of the analyzed profile according to the standard ISO4287.
1 SymRenewTM HPR, produced by Symrise (https://www.symrise.com/scent-and-care/cosmetic-ingredients/, Holzminden)
Results
In order to elucidate and compare the transcriptional regulations of the in cosmetics widely used retinol compared to its newly proposed alternative ingredient, an RNA-Seq was performed on 3D reconstructed epidermis models.
The RNA Seq analysis reveals that the ingredient changes gene expression of 2747 genes compared to the untreated sample. All-trans retinol changes the expression of 3893 genes
compared to the untreated control. From those differentially regulated genes, 2254 genes are regulated by both the ingredient and all-trans retinol (Fig. 1).
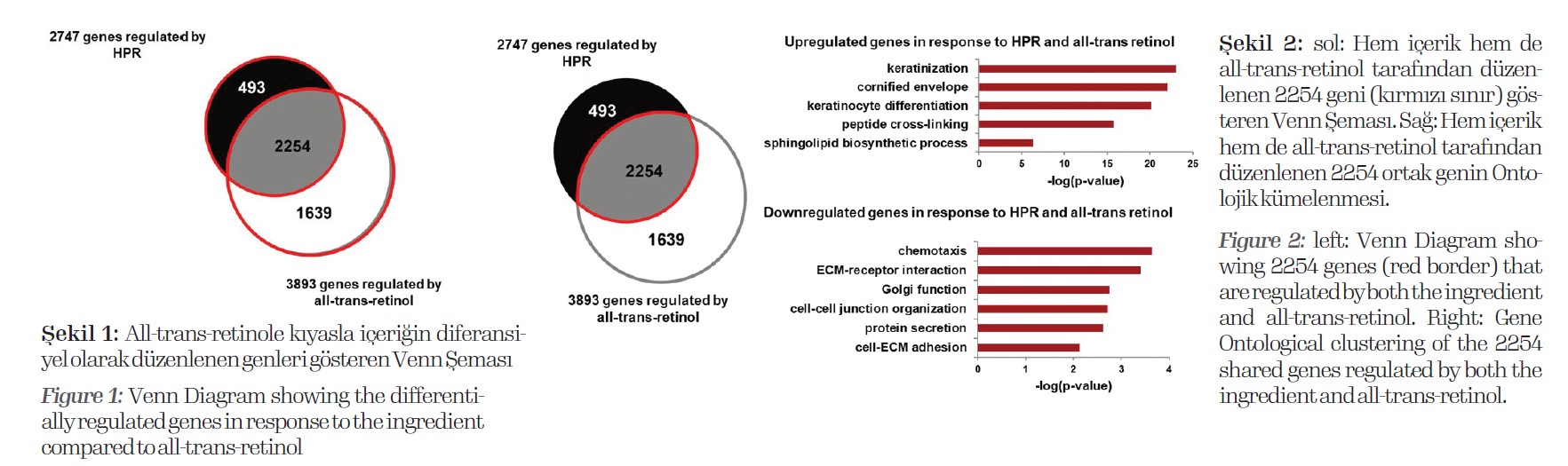
Here, both retinol derivatives induce gene expression, which is associated to
keratinization, cornified envelop, keratinocyte differentiation, peptide cross-linking and sphingolipid biosynthesis, which are processes promoting skin barrier function and epidermal thickening via ceramide production and stratum corneum formation (Fig. 2).
Further, both inhibit gene expression that is linked to processes of chemotaxis, ECM-receptor interaction, Golgi function, cell-cell junction organization, protein secretion. These regulations also indicate an increased keratinization and subsequently an enhanced stratum corneum formation as these processes are repressed there.
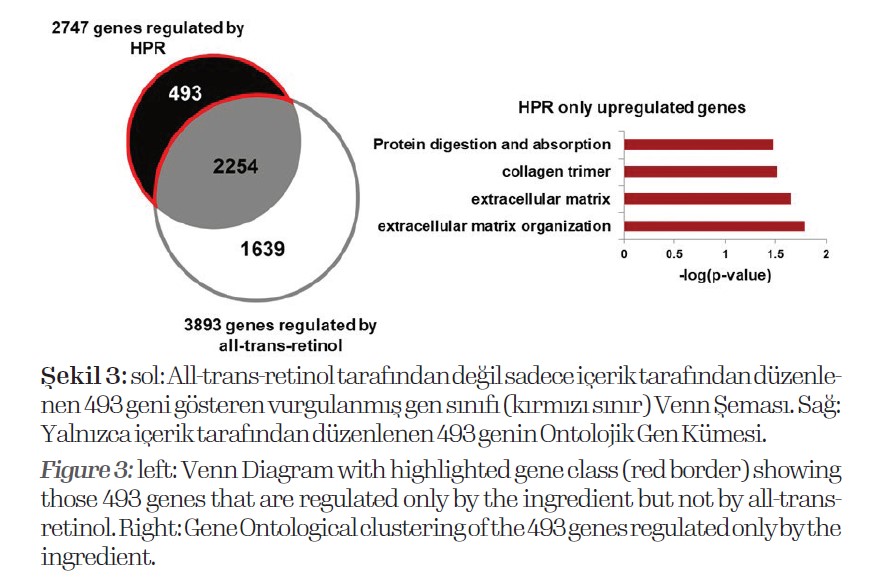
These data show that the gene expression induced by the ingredient differs from that induced by all-trans retinol. In order to show the advantages of the ingredient over alltrans
retinol, we analyzed the 493 genes regulated only by the ingredient but not by all-trans retinol (Fig. 3).
Among those, we found genes that are associated to the upregulation of extracellular matrix organization, extracellular matrix and collagen suggesting improved basement
membrane formation, and dermal-epidermal anchoring.
Further, protein digestion and absorption were promoted, which is very important to protect skin cells from aging processes and improve stratum corneum formation during differentiation. In the next step, we asked if the ingredient is able to improve gene expression compared to all-transretinol.
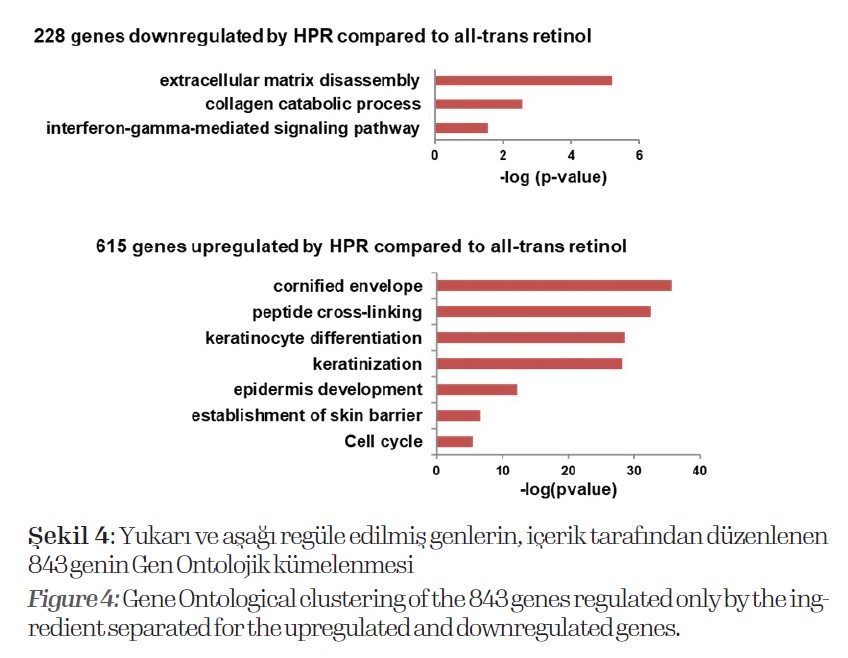
Therefore, the gene expression data of all-trans retinol was used as control for normalization. With this we found 843 genes differentially expressed genes compared to all-trans retinol (Fig. 4).
Here it shows that the ingredient downregulates processes associated with extracellular matrix disassembly, collagen catabolic processes and interferon-gamma signaling. This indicates that the ingredient suppresses extracellular matrix and collagen degradation
and is less irritant than all-trans retinol.
On the other hand, it induces gene expression involved in the cornified envelope, peptide cross-linking, keratinocyte differentiation and cell cycle, among others. Further, we compared gene expression of kallikreins, keratins and further keratinocyte differentiation related genes.
Here for example, Keratin1/10, Keratin 4/13, Filaggrin (Fig. 5) were expressed to a higher extend than in retinol treated samples supporting the gene ontology data. This clearly demonstrates that the ingredient exhibits higher activity with regard to keratinocyte differentiation and thus improves epidermal thickening to a higher extend when used equimolar compared to all-trans retinol.

After elucidating the transcriptomic effects of the ingredient in comparison to all-trans retinol, its phenotypical efficacy was evaluated in a wrinkle in vivo study (Fig. 6). After 14 days of in-home use of all-trans retinol formulation there was a significant reduction (P<0.05) of 7.2%, reaching up to 9.5% in intensity of wrinkles.
It was possible to observe that 100% of the research subjects showed reduction of
wrinkles and expression lines. For the ingredient there was a significant reduction (P<0.05) of 8.5%, reaching up to 11.0% in intensity of wrinkles. It was possible to observe that 94% of the research subjects showed reduction of wrinkles and expression lines.
After 28 days of in-home use of all trans retinol product there was a significant reduction (P<0.05) of 12.6%, reaching up to 15.6% in intensity of wrinkles compared to placebocontrolled skin.
It was possible to observe that 100% of the research subjects showed reduction of wrinkles and expression lines. For the ingredient, there was a significant reduction (P<0.05) of 13.4%, reaching up to 17.0% in intensity of wrinkles compared to placebo-controlled skin.
It was possible to observe that 97% of the research subjects showed reduction of wrinkles and expression lines. These data show the same efficacy for all-trans-retinol and the ingredient in vivo on wrinkle reduction.

Discussion and Conclusion
This study has compared the molecular and phenotypic effects of all-trans retinol and the ingredient, an alternative derivative based on pinacolone esters. Here, we could show that the ingredient shows improved transcriptomic activity on keratinocyte differentiation and
barrier formation when compared to all-trans-retinol.
For example, the ingredient shows advantages over all-trans retinol, as it more increases extracellular matrix organization and collagen formation to strengthen the basal membrane. It seems to be less irritant, as it suppresses interferon mediated signaling.
Further, it exhibits higher activity regarding keratinocyte differentiation and thus improves epidermal thickening to a higher extend when used in equimolar concentrations and compared to all-trans retinol.
In the in vivo wrinkle study the ingredient and all-trans-retinol result in significant wrinkle reduction with similar efficacy. With this, we conclude that the ingredient is a safe and effective alternative anti-aging compound for the use in anti-aging cosmetic formulations.
Bibliography
1 Heyman, R A, Mangelsdorf, D J, Dyck, J Aet al (1992) 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell 68(2) 397-406
2 Levin, A A, Sturzenbecker, L J, Kazmer, Set al (1992) 9-cis retinoic acid stereoisomer binds and activates the nuclear receptor RXR alpha. Nature 355(6358) 359-361
3 Piskunov, A, & Rochette-Egly, C (2012) A retinoic acid receptor RAR pool present in membrane lipid rafts forms complexes with G protein Q to activate p38MAPK. Oncogene 31(28) 3333-3345
4 Masiá, S, Alvarez, S, de Lera, A Ret al (2007) Rapid, Nongenomic Actions of Retinoic Acid on Phosphatidylinositol-3-Kinase Signaling Pathway Mediated by the RetinoicAcid Receptor. Molecular Endocrinology 21(10) 2391-2402
5 Szymański, Ł, Skopek, R, Palusińska, Met al (2020) Retinoic Acid and Its Derivatives in Skin. Cells 9(12) 2660
6 Törmä, H (2011) Regulation of keratin expression by retinoids. Dermato-endocrinology 3(3) 136-140
7 Hubbard, B A, Unger, J G, & Rohrich, R J (2014) Reversal of Skin Aging with Topical Retinoids. Plastic and reconstructive surgery 133(4)
8 Baumann, L (2007) Skin ageing and its treatment. J Pathol 211(2) 241-251
9 Puizina-Ivic, N, Miric, L, Carija, Aet al Modern approach to topical treatment of aging skin. Coll Antropol 34(3) 1145-1153
10 Isoherranen, N, & Zhong, G (2019) Biochemical and physiological importance of the CYP26 retinoic acid hydroxylases. Pharmacology & Therapeutics 204 107400
11 Yin, S, Luo, J, Qian, Aet al (2013) Retinoids activate the irritant receptor TRPV1 and produce sensory hypersensitivity. Journal of Clinical Investigation 123(9) 3941-3951
12 Varani, J, Fay, K, & Perone, P (2007) MDI 301, a non-irritating retinoid, induces changes in human skin that underlie repair. Archives of Dermatological Research
298(9) 439-448
Authors:
Maria Reichenbach, Symrise AG, Germany
Michele Massironi, Cutech Srl, Italy
Mickaël Larnicol, Symrise SAS, France
Translation and Compilation: Tuğba Bayazıt
Application Technologist
Team Leader AMET
Symrise AG

 Here, both retinol derivatives induce gene expression, which is associated to
keratinization, cornified envelop, keratinocyte differentiation, peptide cross-linking and sphingolipid biosynthesis, which are processes promoting skin barrier function and epidermal thickening via ceramide production and stratum corneum formation (Fig. 2).
Further, both inhibit gene expression that is linked to processes of chemotaxis, ECM-receptor interaction, Golgi function, cell-cell junction organization, protein secretion. These regulations also indicate an increased keratinization and subsequently an enhanced stratum corneum formation as these processes are repressed there.
Here, both retinol derivatives induce gene expression, which is associated to
keratinization, cornified envelop, keratinocyte differentiation, peptide cross-linking and sphingolipid biosynthesis, which are processes promoting skin barrier function and epidermal thickening via ceramide production and stratum corneum formation (Fig. 2).
Further, both inhibit gene expression that is linked to processes of chemotaxis, ECM-receptor interaction, Golgi function, cell-cell junction organization, protein secretion. These regulations also indicate an increased keratinization and subsequently an enhanced stratum corneum formation as these processes are repressed there.

 These data show that the gene expression induced by the ingredient differs from that induced by all-trans retinol. In order to show the advantages of the ingredient over alltrans
retinol, we analyzed the 493 genes regulated only by the ingredient but not by all-trans retinol (Fig. 3).
Among those, we found genes that are associated to the upregulation of extracellular matrix organization, extracellular matrix and collagen suggesting improved basement
membrane formation, and dermal-epidermal anchoring.
Further, protein digestion and absorption were promoted, which is very important to protect skin cells from aging processes and improve stratum corneum formation during differentiation. In the next step, we asked if the ingredient is able to improve gene expression compared to all-transretinol.
Therefore, the gene expression data of all-trans retinol was used as control for normalization. With this we found 843 genes differentially expressed genes compared to all-trans retinol (Fig. 4).
Here it shows that the ingredient downregulates processes associated with extracellular matrix disassembly, collagen catabolic processes and interferon-gamma signaling. This indicates that the ingredient suppresses extracellular matrix and collagen degradation
and is less irritant than all-trans retinol.
On the other hand, it induces gene expression involved in the cornified envelope, peptide cross-linking, keratinocyte differentiation and cell cycle, among others. Further, we compared gene expression of kallikreins, keratins and further keratinocyte differentiation related genes.
Here for example, Keratin1/10, Keratin 4/13, Filaggrin (Fig. 5) were expressed to a higher extend than in retinol treated samples supporting the gene ontology data. This clearly demonstrates that the ingredient exhibits higher activity with regard to keratinocyte differentiation and thus improves epidermal thickening to a higher extend when used equimolar compared to all-trans retinol.
These data show that the gene expression induced by the ingredient differs from that induced by all-trans retinol. In order to show the advantages of the ingredient over alltrans
retinol, we analyzed the 493 genes regulated only by the ingredient but not by all-trans retinol (Fig. 3).
Among those, we found genes that are associated to the upregulation of extracellular matrix organization, extracellular matrix and collagen suggesting improved basement
membrane formation, and dermal-epidermal anchoring.
Further, protein digestion and absorption were promoted, which is very important to protect skin cells from aging processes and improve stratum corneum formation during differentiation. In the next step, we asked if the ingredient is able to improve gene expression compared to all-transretinol.
Therefore, the gene expression data of all-trans retinol was used as control for normalization. With this we found 843 genes differentially expressed genes compared to all-trans retinol (Fig. 4).
Here it shows that the ingredient downregulates processes associated with extracellular matrix disassembly, collagen catabolic processes and interferon-gamma signaling. This indicates that the ingredient suppresses extracellular matrix and collagen degradation
and is less irritant than all-trans retinol.
On the other hand, it induces gene expression involved in the cornified envelope, peptide cross-linking, keratinocyte differentiation and cell cycle, among others. Further, we compared gene expression of kallikreins, keratins and further keratinocyte differentiation related genes.
Here for example, Keratin1/10, Keratin 4/13, Filaggrin (Fig. 5) were expressed to a higher extend than in retinol treated samples supporting the gene ontology data. This clearly demonstrates that the ingredient exhibits higher activity with regard to keratinocyte differentiation and thus improves epidermal thickening to a higher extend when used equimolar compared to all-trans retinol.
 After elucidating the transcriptomic effects of the ingredient in comparison to all-trans retinol, its phenotypical efficacy was evaluated in a wrinkle in vivo study (Fig. 6). After 14 days of in-home use of all-trans retinol formulation there was a significant reduction (P<0.05) of 7.2%, reaching up to 9.5% in intensity of wrinkles.
It was possible to observe that 100% of the research subjects showed reduction of
wrinkles and expression lines. For the ingredient there was a significant reduction (P<0.05) of 8.5%, reaching up to 11.0% in intensity of wrinkles. It was possible to observe that 94% of the research subjects showed reduction of wrinkles and expression lines.
After 28 days of in-home use of all trans retinol product there was a significant reduction (P<0.05) of 12.6%, reaching up to 15.6% in intensity of wrinkles compared to placebocontrolled skin.
It was possible to observe that 100% of the research subjects showed reduction of wrinkles and expression lines. For the ingredient, there was a significant reduction (P<0.05) of 13.4%, reaching up to 17.0% in intensity of wrinkles compared to placebo-controlled skin.
It was possible to observe that 97% of the research subjects showed reduction of wrinkles and expression lines. These data show the same efficacy for all-trans-retinol and the ingredient in vivo on wrinkle reduction.
After elucidating the transcriptomic effects of the ingredient in comparison to all-trans retinol, its phenotypical efficacy was evaluated in a wrinkle in vivo study (Fig. 6). After 14 days of in-home use of all-trans retinol formulation there was a significant reduction (P<0.05) of 7.2%, reaching up to 9.5% in intensity of wrinkles.
It was possible to observe that 100% of the research subjects showed reduction of
wrinkles and expression lines. For the ingredient there was a significant reduction (P<0.05) of 8.5%, reaching up to 11.0% in intensity of wrinkles. It was possible to observe that 94% of the research subjects showed reduction of wrinkles and expression lines.
After 28 days of in-home use of all trans retinol product there was a significant reduction (P<0.05) of 12.6%, reaching up to 15.6% in intensity of wrinkles compared to placebocontrolled skin.
It was possible to observe that 100% of the research subjects showed reduction of wrinkles and expression lines. For the ingredient, there was a significant reduction (P<0.05) of 13.4%, reaching up to 17.0% in intensity of wrinkles compared to placebo-controlled skin.
It was possible to observe that 97% of the research subjects showed reduction of wrinkles and expression lines. These data show the same efficacy for all-trans-retinol and the ingredient in vivo on wrinkle reduction.
