Amino-acid based surfactants are widely used in the last two decades in personal care products as well as in household, industrial and institutional cleaners due to a lot of advantages.
They are very mild [1-3] anionic surfactants, which do not lead to dry skin [4] and protect it from the negative influence of severe surfactants [5,6], such as SLS and SLES, by their use as co-surfactants. Additionally to good cleansing properties they show perfect foam formation.
Herewith a high micro-porosity of foam supports its creaminess and stability [7]. Amino-acid based surfactants are readily biodegradable [8-10] and demonstrate extremely low toxicity [11]. These two benefits are very important for creating ecologically pure products without negative impact on environment.
Glutamic acid based surfactants occupy a special position in this range of surface active ingredients because they can be obtained completely from renewable plant sources. This fact enables their application also for COSMOS [12] and NaTrue [13] certified natural personal care products.
Until now, the thickening of formulations based on these “green” surfactants represents the main problem with their application. In this case,
addition of salt does not give the desired effect.
Therefore it was necessary to use water-soluble polymers, such as xanthan gum or acrylates that affects sensory experience and appearance of the final product.
Recently it has been found that, using a combination of sodium salt of cocoyl or lauroyl glutamate with lauryl glucoside, a sufficiently high viscosity of the end product is achieved at a standard surfactant concentration in absence of a thickening agent.
Based on this knowledge, a new product, Rheo2Green, has been developed, ready to be used in mixture concentrations. They simply should be diluted and do not require an addition of further surfactants.
Disodium cocoyl glutamate and lauryl glucoside are “green” surfactants, therefore all products of the Rheo2Green series are COSMOS and NaTrue conform. Both are readily biodegradable and environmentally friendly that makes them suitable for Ecolabel (EU or Nordic Swan) certified end products.
Rheo2Green2 contains sodium benzoate and potassium sorbate in quantities sufficient to preserve end product.

There are outstanding advantages of using Rheo2Green blends as raw materials:
a) The pure lauryl glucoside is a highly viscous liquid, which usually requires pre-heat before use. Rheo2Green concentrates are low viscous and cold processable that can save the necessity of heat boxes in production process.
b) Compared to the use of separate Rheo2Green components, these blends simplify logistic aspects such as storage, purchase and dosage during manufacturing.
c) The price of Rheo2Green raw materials is improved due to production process optimisation.
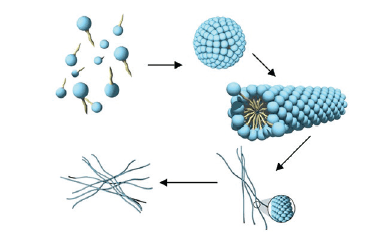
As mentioned above, this combination of surfactants entails a natural micellar thickening. It is typical that most surfactants form spherical micelles. Only under certain conditions wormlike, cylindrical micelles can be build. On a molecular scale these structures look like long polymer molecules. The interaction between these cylindrical micelles provides in a similar manner the thickening effect (Fig1).
 Figure 1
Figure 1: Formation of spherical and cylindrical micelles.
With Rheo2Green products required viscosity is very easy achievable. The concentrate must be diluted with water and instantaneous thickening takes place when pH is adjusted to 4.6-5.3. Figure 2 shows change of viscosity versus pH for the solutions of Rheo2Green1 at different dilution. It has been found that viscosity of these systems has a maximum. For Rheo2Green1 diluted solutions in the absence of further components it is located at constant pH ~4.9. The pH viscosity-maximum can slightly move between 4.5 and 5.3 when additional ingredients are present.
 Figure 2
Figure 2: Viscosity of Rheo2Green1 solutions at different dilution as a
function of pH measured with Brookfield Sp.3, 5rpm, RT. (W%product -
percentage weight of raw material; W %active - corresponding concentration
of active matter).
The impact of different ingredients often used as additives in personal care products on the viscosity of the final formulations has been tested. The results are summarised in Figure 3. Generally, multivalent alcohols as well as preservatives based on sorbate and benzoate can improve (++ or +) viscosity. Other hydrophilic non surface active substances have minor influence on this parameter (+ or 0) up to certain amounts. On the other hand, hydrophobic additives like oils break the viscosity already at low concentration. Surprisingly, polymeric thickening agents destabilize the system and precipitate.

Figure 3: Compatibility of Rheo2Green1 with
typical rinse-off ingredients in terms of homogeneity
and viscosity.
A basic recipe for a shampoo with Rheo2Green2 has been developed (Figure 4). As mentioned before this raw material contains sodium benzoate and potassium sorbate, which act as preservatives in the final product. Challenge test according to Ph. Eur. (preparation for cutaneous use) and ISO 11930 confirms that the amount is sufficient for a complete microbiological protection of the end product when 30% Rheo2Green2 is used. The results correspond to criteria A.
 Figure 4:
Figure 4: ”Green Thickened Shampoo” based on Rheo2Green2
The scope of application for Rheo2Green products is very wide: from skin and hair care products to household and animal care. Everywhere where mildness, outstanding foam and good cleaning are necessary Rheo2Green is recommended. Achievement of viscosity without using thickening agents, excellent flow behavior and transparency are additional advantages of formulations based on Rheo2Green concentrates. Such low pH personal care products are able to decrease pH of unbalanced skin to the healthy neutral 5.5 level. They can be produced cost-effective, are environmentally friendly and suitable for “Natural/Green” certification.
- References
1. Kanari M, Kawasaki Y, Sakamoto K. J. Soc. Cosm. Chem. 1993; 27: 498.
2. Nnanna IA, Xia J. Protein-Based Surfactants. Surfactant Science Series 2001; 101: 261-270.
3. Husmann M, Weisse J, Wragg P, Wasko J. Perlastan Surfactants Derived from Naturally-occurring Amino Acids. Cosmetic Science Technology
2008; 194-201.
4. Held E, Husmann M, Heinrich U, Tronnier H. Dermatological Compatibility of Amino Acid Based Surfactants – A Clinical Trial. SOFW Journal 2011;
137: 38-42.
5. Lee CH, Kawasaki Y, Maibach HI. Effect of Surfactant Mixtures on Irritant Contact Dermatitis Potential in Man: Sodium Lauroyl Glutamate and
Sodium Lauryl Sulphate. Contact Dermatitis 1994; 30: 205-209.
6. Sugar M, Schmukker R. Reduzierung der Hautadsorption von Sodium Laureth Sulfat: Ein neuer Weg, die Hautfeuchtigkeit nach Anwendung von
Duschprodukten zu erhöhen. SOFW Journal 2001; 127: 3-5.
7. Scherbakow S, Husmann M, Bykov Y, Kirchner T, Stäuble C, Wragg P. Dynamic Properties of Amino Acid Based Surfactants. Personal Care Europe
September 2016;
8. Gitte I. Petersen og Trine Thorup Anderse, DHI – Vand og Miljø, Arbejdsrapport fra Miljøstyrelsen Nr. 10 2004: Substitution af overflade aktive
stoffer i kosmetiske produkter.
9. Uusitalo T (Ecolabelling Finland), Hirsch T (Ecolabelling Norway), Eskeland M B (Ecolabelling Norway). Revision of the harmonised Detergent
Ingredient Database; Final report for DID 2016, Version 2016, May 2016.
10. Husmann M. Surface Active Agents Derived from Naturally-Occurring Amino Acids. CESIO - 7th World Surfactants Congress 2008.
11. ECHA website: https://echa.europa.eu/nl/registration-dossier/-/registered-dossier/11409
12. COSMOS website: http://www.cosmos-standard-rm.org/
13. NATRUE website: http://www.natrue.org/information-for/manufacturers/raw-materials/
 There are outstanding advantages of using Rheo2Green blends as raw materials:
a) The pure lauryl glucoside is a highly viscous liquid, which usually requires pre-heat before use. Rheo2Green concentrates are low viscous and cold processable that can save the necessity of heat boxes in production process.
b) Compared to the use of separate Rheo2Green components, these blends simplify logistic aspects such as storage, purchase and dosage during manufacturing.
c) The price of Rheo2Green raw materials is improved due to production process optimisation.
As mentioned above, this combination of surfactants entails a natural micellar thickening. It is typical that most surfactants form spherical micelles. Only under certain conditions wormlike, cylindrical micelles can be build. On a molecular scale these structures look like long polymer molecules. The interaction between these cylindrical micelles provides in a similar manner the thickening effect (Fig1).
There are outstanding advantages of using Rheo2Green blends as raw materials:
a) The pure lauryl glucoside is a highly viscous liquid, which usually requires pre-heat before use. Rheo2Green concentrates are low viscous and cold processable that can save the necessity of heat boxes in production process.
b) Compared to the use of separate Rheo2Green components, these blends simplify logistic aspects such as storage, purchase and dosage during manufacturing.
c) The price of Rheo2Green raw materials is improved due to production process optimisation.
As mentioned above, this combination of surfactants entails a natural micellar thickening. It is typical that most surfactants form spherical micelles. Only under certain conditions wormlike, cylindrical micelles can be build. On a molecular scale these structures look like long polymer molecules. The interaction between these cylindrical micelles provides in a similar manner the thickening effect (Fig1).
 Figure 1: Formation of spherical and cylindrical micelles.
With Rheo2Green products required viscosity is very easy achievable. The concentrate must be diluted with water and instantaneous thickening takes place when pH is adjusted to 4.6-5.3. Figure 2 shows change of viscosity versus pH for the solutions of Rheo2Green1 at different dilution. It has been found that viscosity of these systems has a maximum. For Rheo2Green1 diluted solutions in the absence of further components it is located at constant pH ~4.9. The pH viscosity-maximum can slightly move between 4.5 and 5.3 when additional ingredients are present.
Figure 1: Formation of spherical and cylindrical micelles.
With Rheo2Green products required viscosity is very easy achievable. The concentrate must be diluted with water and instantaneous thickening takes place when pH is adjusted to 4.6-5.3. Figure 2 shows change of viscosity versus pH for the solutions of Rheo2Green1 at different dilution. It has been found that viscosity of these systems has a maximum. For Rheo2Green1 diluted solutions in the absence of further components it is located at constant pH ~4.9. The pH viscosity-maximum can slightly move between 4.5 and 5.3 when additional ingredients are present.
 Figure 2: Viscosity of Rheo2Green1 solutions at different dilution as a
function of pH measured with Brookfield Sp.3, 5rpm, RT. (W%product -
percentage weight of raw material; W %active - corresponding concentration
of active matter).
The impact of different ingredients often used as additives in personal care products on the viscosity of the final formulations has been tested. The results are summarised in Figure 3. Generally, multivalent alcohols as well as preservatives based on sorbate and benzoate can improve (++ or +) viscosity. Other hydrophilic non surface active substances have minor influence on this parameter (+ or 0) up to certain amounts. On the other hand, hydrophobic additives like oils break the viscosity already at low concentration. Surprisingly, polymeric thickening agents destabilize the system and precipitate.
Figure 2: Viscosity of Rheo2Green1 solutions at different dilution as a
function of pH measured with Brookfield Sp.3, 5rpm, RT. (W%product -
percentage weight of raw material; W %active - corresponding concentration
of active matter).
The impact of different ingredients often used as additives in personal care products on the viscosity of the final formulations has been tested. The results are summarised in Figure 3. Generally, multivalent alcohols as well as preservatives based on sorbate and benzoate can improve (++ or +) viscosity. Other hydrophilic non surface active substances have minor influence on this parameter (+ or 0) up to certain amounts. On the other hand, hydrophobic additives like oils break the viscosity already at low concentration. Surprisingly, polymeric thickening agents destabilize the system and precipitate.
 Figure 3: Compatibility of Rheo2Green1 with
typical rinse-off ingredients in terms of homogeneity
and viscosity.
A basic recipe for a shampoo with Rheo2Green2 has been developed (Figure 4). As mentioned before this raw material contains sodium benzoate and potassium sorbate, which act as preservatives in the final product. Challenge test according to Ph. Eur. (preparation for cutaneous use) and ISO 11930 confirms that the amount is sufficient for a complete microbiological protection of the end product when 30% Rheo2Green2 is used. The results correspond to criteria A.
Figure 3: Compatibility of Rheo2Green1 with
typical rinse-off ingredients in terms of homogeneity
and viscosity.
A basic recipe for a shampoo with Rheo2Green2 has been developed (Figure 4). As mentioned before this raw material contains sodium benzoate and potassium sorbate, which act as preservatives in the final product. Challenge test according to Ph. Eur. (preparation for cutaneous use) and ISO 11930 confirms that the amount is sufficient for a complete microbiological protection of the end product when 30% Rheo2Green2 is used. The results correspond to criteria A.
 Figure 4: ”Green Thickened Shampoo” based on Rheo2Green2
The scope of application for Rheo2Green products is very wide: from skin and hair care products to household and animal care. Everywhere where mildness, outstanding foam and good cleaning are necessary Rheo2Green is recommended. Achievement of viscosity without using thickening agents, excellent flow behavior and transparency are additional advantages of formulations based on Rheo2Green concentrates. Such low pH personal care products are able to decrease pH of unbalanced skin to the healthy neutral 5.5 level. They can be produced cost-effective, are environmentally friendly and suitable for “Natural/Green” certification.
Figure 4: ”Green Thickened Shampoo” based on Rheo2Green2
The scope of application for Rheo2Green products is very wide: from skin and hair care products to household and animal care. Everywhere where mildness, outstanding foam and good cleaning are necessary Rheo2Green is recommended. Achievement of viscosity without using thickening agents, excellent flow behavior and transparency are additional advantages of formulations based on Rheo2Green concentrates. Such low pH personal care products are able to decrease pH of unbalanced skin to the healthy neutral 5.5 level. They can be produced cost-effective, are environmentally friendly and suitable for “Natural/Green” certification.