GEA introduces the kytero® 10, the world's smallest single use disk stack centrifuge. This mini centrifuge is used in the biopharmaceutical, food and new food industries for product development, which can often only provide small bioreactor sizes.
Its task is the separation of bacteria, cell cultures and yeasts as well as applications in cell and gene therapy. The kytero® 10 is the smallest model in the GEA range for cell separation and recirculation and is suitable for volumes from one to 10 liters. Thanks to the low shear design, high cell viability with continuous cell harvesting and perfusion processes, by use of centrifugal separation, is possible from laboratory scale up to production size. The kytero® 10 separator is ideal for the smallest batch and perfusion fermenters.
Test users: upscaling makes it possible to overcome previous limits
Other models of the kytero® series cover ranges from 500 to 2,000 liters (batch).
Test users have found that this concept overcomes previous limitations. The results achieved can be scaled up to larger model variants and industrial production with classic stainless steel separators, which facilitates process transfer and validation.
Single use offers high safety against contamination
The proven GEA disc stack centrifuge technology has been implemented in compact machines with easy-to-handle units that contain all parts that come into contact with the product. These units are exchanged after a production run, providing maximum safety against contamination. Gamma-treated exchangeable units are available as standard.
No complex CIP and SIP cleaning and innovative drive system
The new single use perfusion separators offer the same advantages as classic disk stack separators, but without the need for cleaning processes (CIP (cleaning-in-place) and SIP (sterilization-in-place). They are ready for the next process run in just a few minutes and require no media other than electricity and air. The compact design and the easy and self-explanatory operability enable simple operation in any system. The non-contact drive system breeze Drive® ensures safe operation under high biocontainment requirements.
First single use disk stack centrifuge for perfusion on a laboratory scale
The new kytero® 10 separators enable continuous operation over the perfusion period and continuous clarification of the fermentation broth. The clarified liquid, which usually contains the product, is continuously removed from the process and fed to the next process stages. Exceptions are bacterial processes and new food applications, where the cells are the valuable product. The concentrated biomass is gently returned to the bioreactor, ensuring high vitality and productivity.
Continuous processing reduces costs
Continuous processing reduces the size of the bioreactor and significantly lowers costs. Operators no longer have to wait for the end of a batch run to separate cells and recover the target protein. Instead of discarding or harvesting cells at the end, they are returned to the bioreactor and only discharged partially so that production can run continuously for weeks. This makes it possible to bring new products to market faster and more cost-effectively.
The important role of kytero® 10 in the production of vaccines and mAbs
GEA developed the kytero® 10 separators to provide the biopharmaceutical industry with robust and scalable upstream processes. The demand for vaccines and monoclonal antibodies (mAbs) is continuously increasing. These separators are designed for high-intensity, continuous cell separation and round off the kytero® series at the bottom. Monoclonal antibodies are successfully used for the treatment of cancer and other serious diseases.
Pre-tests for the GEA kytero® 500 setup
Prior to the main cell centrifugation test, a pre-investigation spin test of cell concentration and turbidity was performed using the Hettich ROTOFIX centrifuge. The centrifuge was used at a setpoint of 2000 xg and the cell suspension was centrifuged with 5 different spin times. At 2000 xg, the g-force is in a similar range to that of the GEA kytero® 500, allowing the initial parametrization (bowl speed rate, feed flow rate) to be carried out based on the required spinning time and relative centrifugal force. The data is shown in Table 1-1.
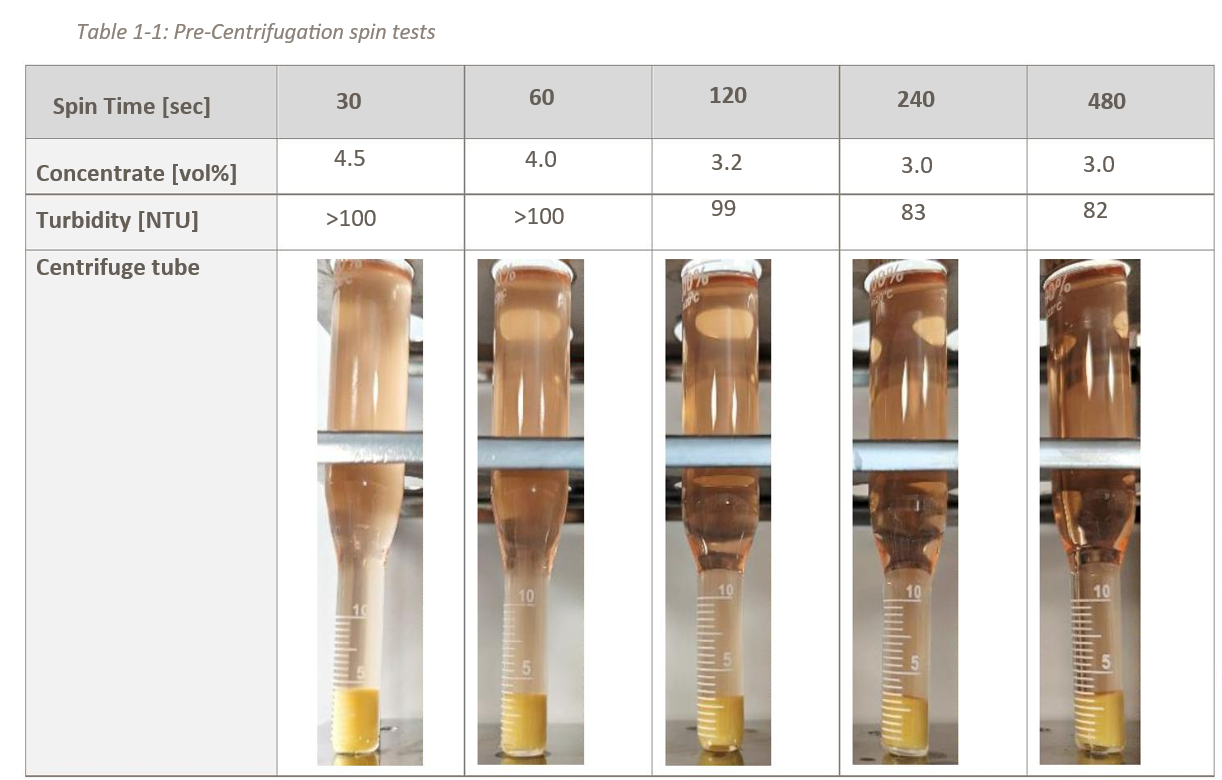
Conclusion of the pre-spin-test was that a high cell concentration (=Concentrate [vol%] in Table 1-1, means cell pellet to total volume ratio) and the lowest turbidity can be achieved with longer spin times (correlates with lower feed flow rates for the GEA kytero® 500). However, many small particles were part of the raw bulk which can only be reduced to a certain level by higher spinning times. Thus, a target turbidity limit of ~80 NTU was determined.
No product loss was observed during centrifugation and no negative effects on harvest bulk pH, DNA-concentration or product quality were observed for the tested settings.
The GEA kytero® single-use pharma separator ensures a high cell harvest step yield and process safety without any cell mass limitations and is therefore an excellent instrument for high quality GMP compliant harvest bulk generation in biotechnology and biopharmaceutical industry.
For more information you can visit below web site:
https://gea.com/kytero